For all you stoners who didn't pay attention in organic chemistry, here's a second chance to learn how to do something that you would have already known if you'd stayed awake. I'm not recommending you do this. And it's not like the Manhattan Project; this information is online in various forms. Rather, it's simple organic chemistry - called a cyclization - where a molecule of CBD oil, aka cannabidiol, under certain sets of conditions, can be coaxed to forming an entirely different chemical compound - THC, one of the psychotropic chemicals in marijuana.
Admit it, the only reason that you guys are all reading this is that you think I'm going tell you how to convert CBD oil, which is not illegal or psychotropic (1,2) and can pretty much be obtained in every store in Manhattan, into THC, which is both illegal and psychotropic. "Hmm," those of you who haven't already nuked every brain cell are thinking," why can't I just get a whole bunch of CBD and make my own THC?"
Well, you can, and that's where organic synthesis - the art of converting one organic (3) molecule into another by one or more chemical reactions - comes in handy. Anyone in the mood for a lesson?
LESSON 1 - NATURE LOVES 6-MEMBERED RINGS
Organic compounds that contain 6-membered rings exceedingly common, both in nature and synthetically. The atoms most commonly found in 6-membered ring compounds are carbon, nitrogen, and oxygen. Six is a magic number in organic chemistry. You don't want to know why. Here are a few examples (Figure 1).

Figure 1. Some examples of naturally occurring organic chemicals containing 6-membered rings.
There is an infinite number of synthetic organic molecules with 6-membered rings out there as well. Some examples - DDT, dioxin, TNT, aspirin, Valium.
LESSON 2- MOLECULES THAT CAN FORM 6-MEMBERED RINGS ARE HIGHLY MOTIVATED TO DO SO
So, let's look at the structures of CBD side by side with THC and see how close they really are, and also why CBD is experiencing an inferiority complex.
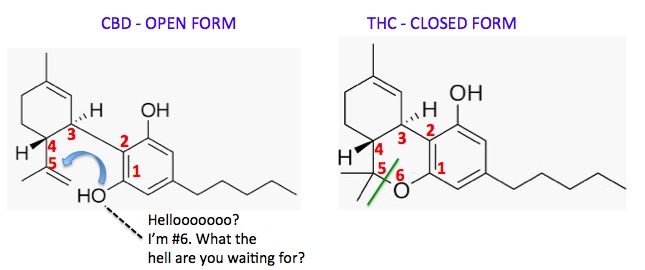
Figure 2. CBD (Left) and THC are isomers - they have the same chemical formula C21H30O2 but different structures and chemical and pharmacological properties. To convert CBD (open form, Left) to THC (closed form) the hydroxyl group (blue arrow) needs to form a bond to carbon #5. This new bond is shown (Right) as a green line. The oxygen atom becomes the sixth member of the new ring.
LESSON THREE - CAN YOU MAKE THE 5-6 BOND FORM, CONVERTING CBD INTO THC?
Yes. Any organic chemist will look at this simple problem and tell you that this reaction will be catalyzed by acid. Depending on who you ask you might get a wide variety of answers, such as:
- Hydrochloric acid
- Sulfuric acid
- p-Toluenesulfonic acid
- Boron tribromide
- Acetic acid (vinegar)
All of these are "correct" in that they will perform this conversion, at least to some extent. But if you're going to be smoking whatever you're cooking up in your kitchen the term "to some extent" comes into play.
LESSON FOUR - ORGANIC REACTIONS CAN BE VERY DIRTY
Perhaps the best measure of the success of a chemical reaction in organic chemistry is the yield. This is simply the amount of pure, isolated material that goes in a bottle divided by the theoretical amount that should be formed if the reaction were perfect. Perfect reactions are rare. A yield of 100% is usually greeted with suspicion. No matter how good a given reaction, byproducts (impurities) almost always form. Every gram of byproduct reduces the amount of final material in the bottle. A yield of 90% is excellent, 70% is fair, 50% is pretty bad, and below that is a horror show. Low yielding reactions typically have multiple impurities. This is why you should not make your own THC.
There are a number of literature examples of people trying to do just this. What appears on paper to be a simple transformation is, in reality, a big mess.
Watanabe, et. al.al., used simulated gastric fluid (SGF, basically salty hydrochloric acid, either with or without a digestive enzyme) to see whether this conversion could take place in the stomach (it probably doesn't). After 20 hours - far longer than any drug would stay in the stomach - only 2.9% of the CBD was converted to THC. Three other cannabinoids were forms in yields ranging from 1-10%. The rest? Who knows? Depending on the reaction conditions, dozens, perhaps hundreds, of byproducts, could be formed. Most of them are unknown and could be dangerous. (For the quintessential example of how even minor deviations from a known procedure can ruin lives see Frozen Addicts, Garage Drugs And Funky Brain Chemistry).
Merrick, et. al., investigated the psychoactive components of CBD after it was exposed to SGF. Using HPLC (4), a standard method of determining the purity of a reaction mixture, the group identified two THC isomers, unreacted CBD, and two unknown impurities (Figure 2). Keep in mind that even this reaction, which was run under carefully controlled conditions gave a mixture of five chemicals. There are almost certainly more in there that haven't been identified.
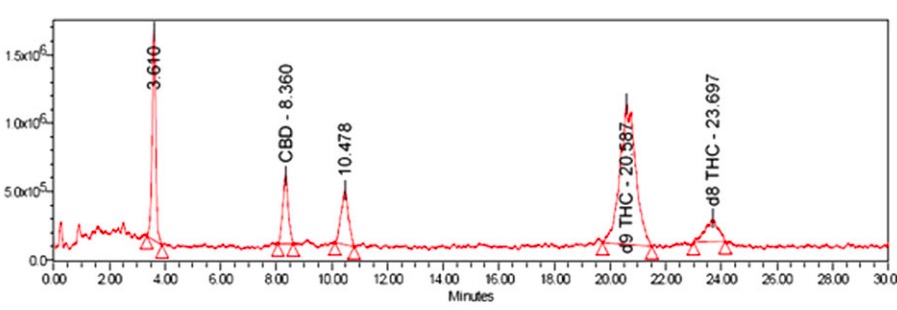
Figure 2. HPLC trace of psychoactive substances formed by reacting CBD with SGF. Source: Cannabis and Cannabinoid Research Volume 1.1, 2016 DOI: 10.1089/can.2015.0004
An example of something even worse is shown in Figure 3. The research chemical supplier Sigma-Aldrich extracted the cannabinoids from a pot brownie and got this collection of 11 different chemicals some CDB-like and some THC-like. Although this mixture did not result from the reaction of CBD with acid, it shows how complex marijuana chemistry can be.
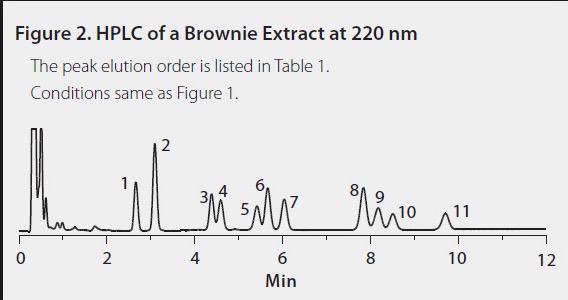
Figure 3. HPLC trace of pot brownie chemicals. Each peak represents a different chemical compound. Source: Sigma-Aldrich
UP TO YOU
So, if you feel sufficiently empowered by this article to put on your lab coat and try your luck I wish you plenty of it. Organic chemistry isn't always predictable. On paper, it looks like you could sneeze on CBD and have all the THC your heart desires. In the lab, things are quite different. In your kitchen - forget it.
NOTES:
(1) Psychotropic drugs produce altered mental states.
(2) CBD may not pack the punch of THC, but this doesn't mean it's inert. In fact, Epidiolex - pure CBD - is approved by the FDA for treating certain seizures in children younger than two years. It also may or may not be legal depending on how much THC is in it. The whole thing is a giant mess.
(3) What chemists mean by organic is quite different than what is used for foods. See my op-ed in the Chicago Tribune Organic Oreos? A chemist explains what 'organic' really means
(4) HPLC stands for High-Performance Liquid Chromatography - a very powerful analytical method for determining the composition of mixtures or the purity of single compounds.