
Just what pain patients didn't need: Another useless substitute for real pain medication. This time, it's the wildly overhyped CBD (cannabidiol) oil that took the hit, courtesy of a new paper in the Journal of Pain in which Andrew Moore and colleagues from the University of Alberta conducted a meta-analysis of 16 randomized controlled clinical trials where the drug was tested against various pain-related conditions. Unsurprisingly, it pretty much bombed with one possible exception. The title alone, Cannabidiol (CBD) Products for Pain: Ineffective, Expensive, and With Potential Harms, speaks volumes.
No one should be surprised that CBD didn't make the cut as a pain therapy. The pseudo-drug has been claimed to have utility for nearly every malady suffered by humans and other beasts. In his article, Moore comments on the ubiquity of unsubstantiated claims (1) made about CBD. (There are claims for at least 10 different conditions that can be found on Twitter alone.)
[Online] outlets often remain unchecked and unbalanced and appear to be aimed at promoting revenue rather than safe practice. Consumers (people living with pain), their careers, and their professional advisers need more balanced, evidence-informed consumer advice. That can now be provided.
Moore, et. al., The Journal of Pain
Furthermore, the authors expressed skepticism about the contents of the bottle, a concern I have addressed multiple times about dietary supplements."
The U.S. analysis of 105 products found that only 1 in 4 products were accurately labeled for CBD, 1 in 5 had less than 90% of the advertised CBD, and 1 in 2 had more than 110%. The range indicated that CBD content varied from almost nothing to very large amounts.
Nice.
Meta-analysis of RCTs
The group searched PubMed and ClinicalTrials.gov for randomized, double-blind trials conducted since 2019 in which CBD (at any dose and through any means of administration) was compared to a placebo for any painful condition. Sixteen trials met these criteria.
Results: CBD has little or no effect on pain
Below is a brief summary (Table 1) of trials included in the meta-analysis and their results.
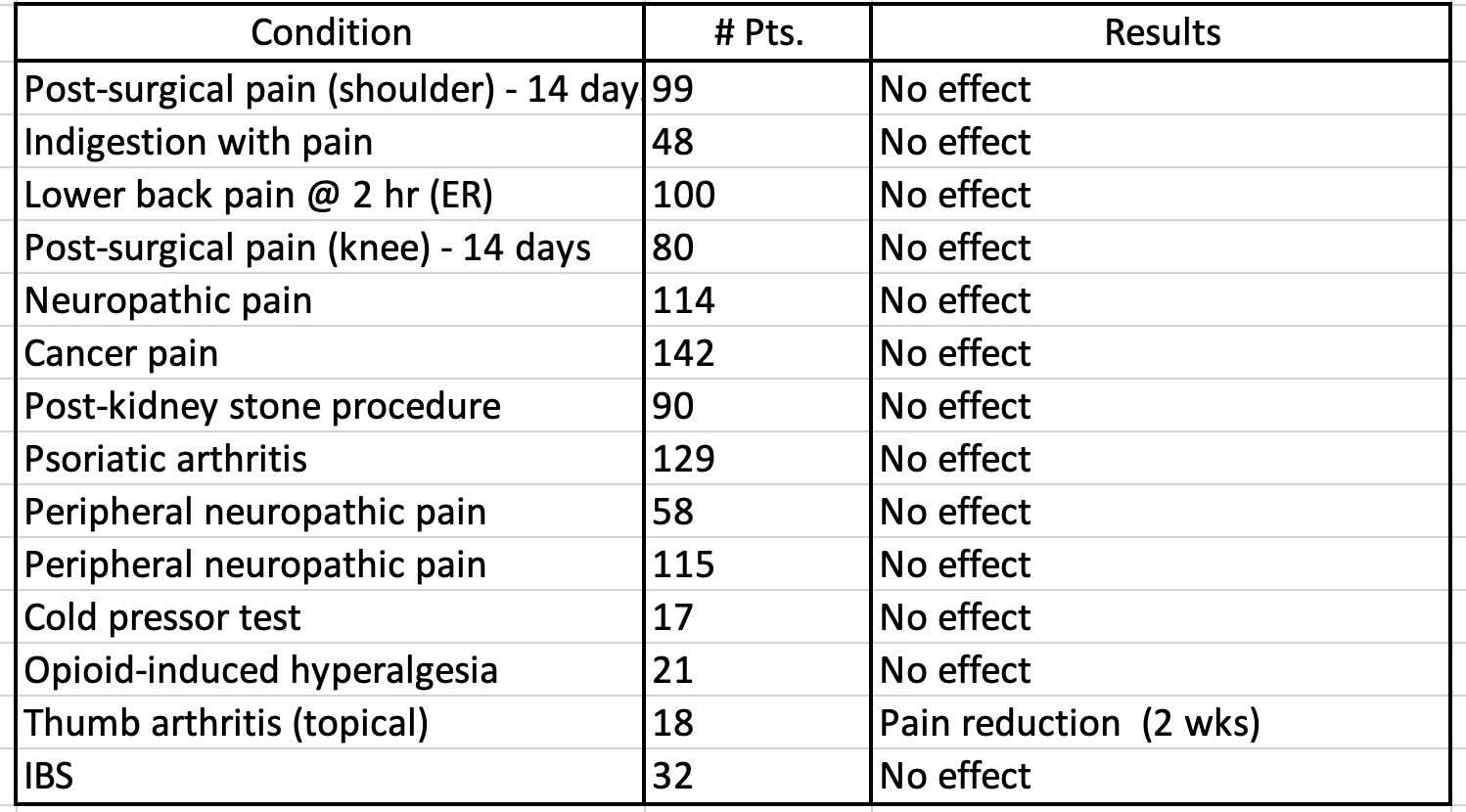
We can graphically visualize these results in Table 1 – a Forest plot.
Source: Moore et al., The Journal of Pain, Vol 25, No. 4 (April), 2024: pp 833–842.
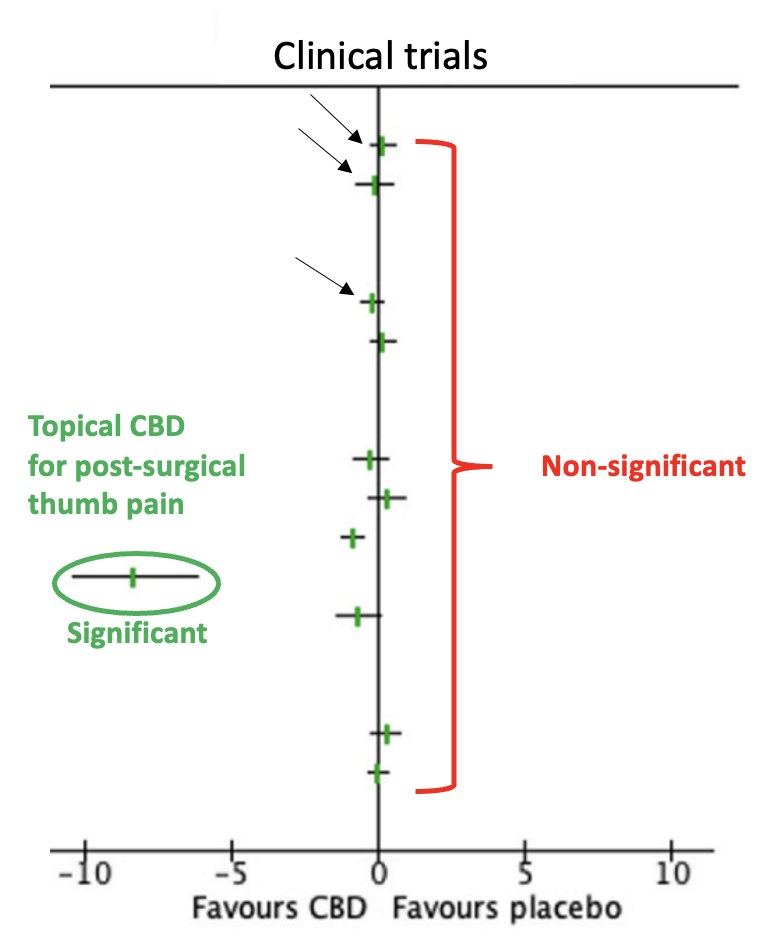
Figure 1. A Forest plot of the 16 trials that met the meta-analysis criteria. The area to the left of the vertical zero line indicates trials where CBD outperformed placebo (one). The area to the right indicates trials where placebo outperformed CBD (none). When a hatch-mark touches (or crosses) the vertical zero line, the results are not significant. Only one (green oval) of 16 trials – topical CBD oil for arthritic thumb pain – showed a significant advantage of CBD over placebo, but it should be noted that this trial had only 18 participants.
Summary
Moore and colleagues not only demonstrated the lack of utility of CBD but also opined that the drug is essentially being given a free pass and that this will ultimately harm people suffering from pain.
[It is unclear] why there is tolerance for the marketing and use of a product without proven benefit but with risk of harm to a large population of people suffering from debilitating pain. This may be due to a misplaced perception of safety [or] a desire of governments to create markets in what is perceived as a new area for national gross domestic product (GDP) growth... What we do know is that if we collude in pretending that we have treatments, we are not facing up to the need for investment in analgesic discovery and innovation. It is sobering to reflect that changes to state medical cannabis laws in the United States to allow greater use have had no important impact on the rate of opioid or nonopioid prescribing or procedures. [emphasis added]
In other words, CBD joins the growing list of drugs that are either ineffective in treating pain, potentially dangerous, or both. But as long as opioids are not used, I guess that's OK.
NOTE:
(1) There is a pharmaceutical-grade CBD (brand name Epidiolex), which is approved only for seizures.



