
The FDA just pulled the plug on brominated vegetable oil (BVO) – a thoroughly unnecessary food additive. While the chances of this decision either helping or harming anyone are slim, it's still the right decision. I will discuss why the chemical has been used and why it may have a small risk (and no benefit), and I will throw in a Dreaded Chemistry Lesson From HellTM showing why it did its job – keeping citrus-flavored sodas homogeneous.
BVO is an emulsifying agent
As we all know, oil and water don't mix. The citrus flavors (most of which are naturally occurring) added to flavor soda are oils that are lighter than water. Here are two flavors with their water solubility and densities. (The density of water is 1.0 grams/mL)
Orange flavor
- Octyl acetate: Insoluble in water, density = 0.87
- Ethyl butyrate: Insoluble in water, 0.88
- d- Limonene: Insoluble in water, 0.81
Lemon flavor
- Citral: Insoluble in water, 0.89
- d- Limonene: Insoluble in water, 0.88
- Linalool: Slightly soluble in water, 0.86
The primary essential oils that give fruity soda its flavor (1) are insoluble in and lighter than water. If not for BVO, you'd have a situation like this, where the oil collects on the top.

Oil floating on water. Image: Wikipedia Commons
Smoothing things out: Emulsions
To get around the oil-water problem soda manufacturers used BVO to make an emulsion - a homogeneous mixture.
ChatGPT: "An emulsion is a mixture of two immiscible liquids, where one liquid is dispersed as tiny droplets within the other. This dispersion is typically stabilized by an emulsifying agent, which prevents the droplets from coalescing and separating. Common examples include milk (an emulsion of fat in water) and mayonnaise (an emulsion of oil in vinegar or lemon juice)."
Of course, you could make a temporary emulsion by shaking the hell out of the soda bottle (like you might with oil and vinegar) before opening it (make sure you hand it to some sucker to open). Alternatively, something like BVOs will work. But not VOs.
Vegetable oils don't work
If soda manufacturers were to try to use plain old stinky vegetable oil for emulsification, it wouldn't do the job. For example, corn oil, is a complex mixture of vegetable oils having a density of 0.92 g/mL, so adding this to the citrus oils would make no difference; the corn and citrus oils would dissolve together and remain layered on top of the water. However, the primary component of corn oil is linoleic acid, an unsaturated fatty acid containing two double bonds, which provides a chemical handle to modify the molecule. This is where the bromine comes in.
To explain this, we need some help from our hell-bound friends Steve and Irving, your loyal but always cantankerous hosts of The Dreaded Chemistry Lesson From HellTM. They were on vacation in powerless, 100o Houston but left because it was too chilly. Let's welcome them back!

Steve (right) and Irving return from a meh vacation in Houston. It was a bit chilly for them, and it would seem that the Astros – barely a .500 team – don't do so well when they're not cheating (2). Of course, they can always switch and become Yankee fans – a different type of hell.
Bromination of linoleic acid
The figures below should explain why BVOs were used in the first place. Alkenes (compounds having at least one carbon-carbon double bond) react quickly with bromine to give dibromoalkanes:
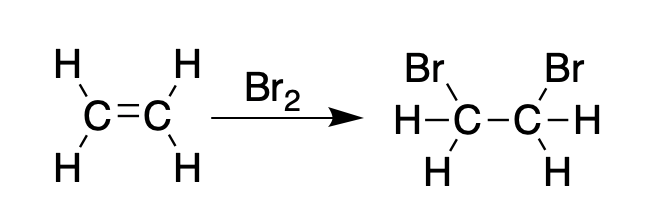
Ethylene reacts with bromine to form 1,2-dibromoethane.
Likewise, linoleic acid reacts with 2 molecules of bromine to form 9,10,12,13-tetrabromooctadecanoic acid, a brominated vegetable oil.
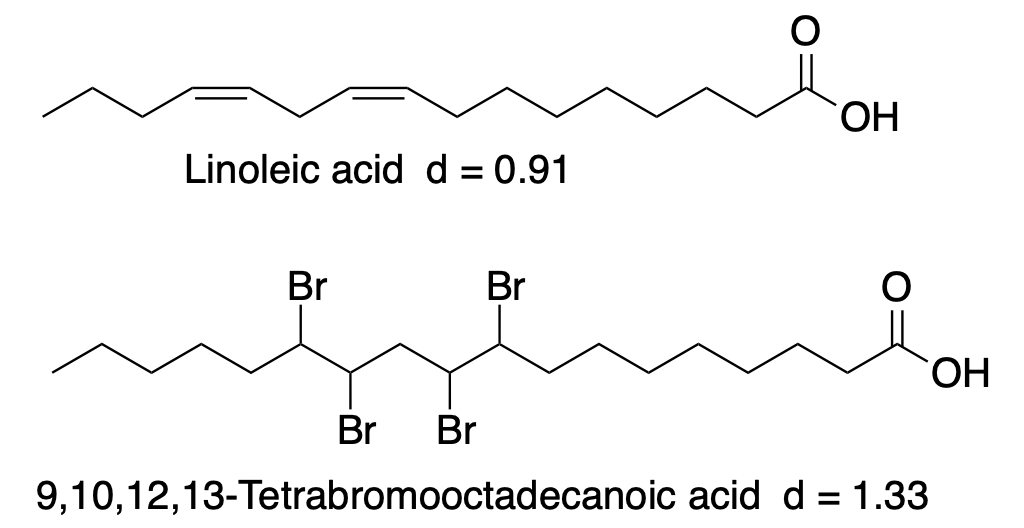
Why do this?
Linoleic acid, like citrus oils, is lighter than water, with a density of 0.92 g/mL. However, bromine and organobromine derivatives are usually quite dense. The following examples provide evidence of this:
- Molecular bromine (Br2), with a density of 3.1 g/mL (this means that 1 mL of bromine weighs 3.1 grams) is the second densest non-metallic element (3). To put this in perspective for non-chemists, a 1-liter bottle of bromine weighs 7 pounds compared to 2.3 pounds for a liter of milk.
- Methylene chloride, CH2Cl2, has a density of 1.7 while methylene bromide, CH2Br2, is 50% denser (2.5).
- Carbon tetrachloride (CCl4), occasionally used as a solvent, is rather dense (1.6 g/mL). It often comes in a 4-liter bottle, and these bad boys weigh 13 pounds. But 4 liters of carbon tetrabromide (CBr4), a solid that is (fortunately) rarely used, weighs a spine-mangling 30 pounds.
It should not be surprising that chemically adding bromine to corn oil makes it denser (heavier), which is why BVOs have been used in certain sodas. When BVOs are added to citrus soda, there is still a mixture of water plus water-insoluble oils, but the oil mixture now will have a density closer to that of water. The oils and water still won't dissolve in each other, but since the two components have a similar density, neither is especially motivated to go racing to the top of the bottle, resulting in a stabilized emulsion. This is why sodas like Fresca and Mountain Dew are cloudy (4).
Health hazard of BVOs, if any
I'm not going to go into detail here, but a paper (outline only) in the journal Food and Toxicology discusses the effects of BVO on Sprague-Dawley rats. Unlike most chemical scare papers, this one is at least believable, showing a dose-dependent effect of BVO metabolites on certain hormones in both male and female rats. (Quiz: the hormones affected are thyroid hormones. Why?) Does it do the same in humans? Impossible to say but it's now a moot point.
Substitutes
Harmful or not, BVOs are unnecessary. There are other emulsifying agents that can be used in its place. One of these is called sucrose acetate hexaisobutyrate (SAH), and it is so non-toxic that symptoms, primarily gastrointestinal, don't show up in rats until they are fed more than 10 grams, roughly 2-4% of the animal's body weight. For humans, that's about 5 pounds. You try eating 5 pounds of anything, and let's see how you feel.
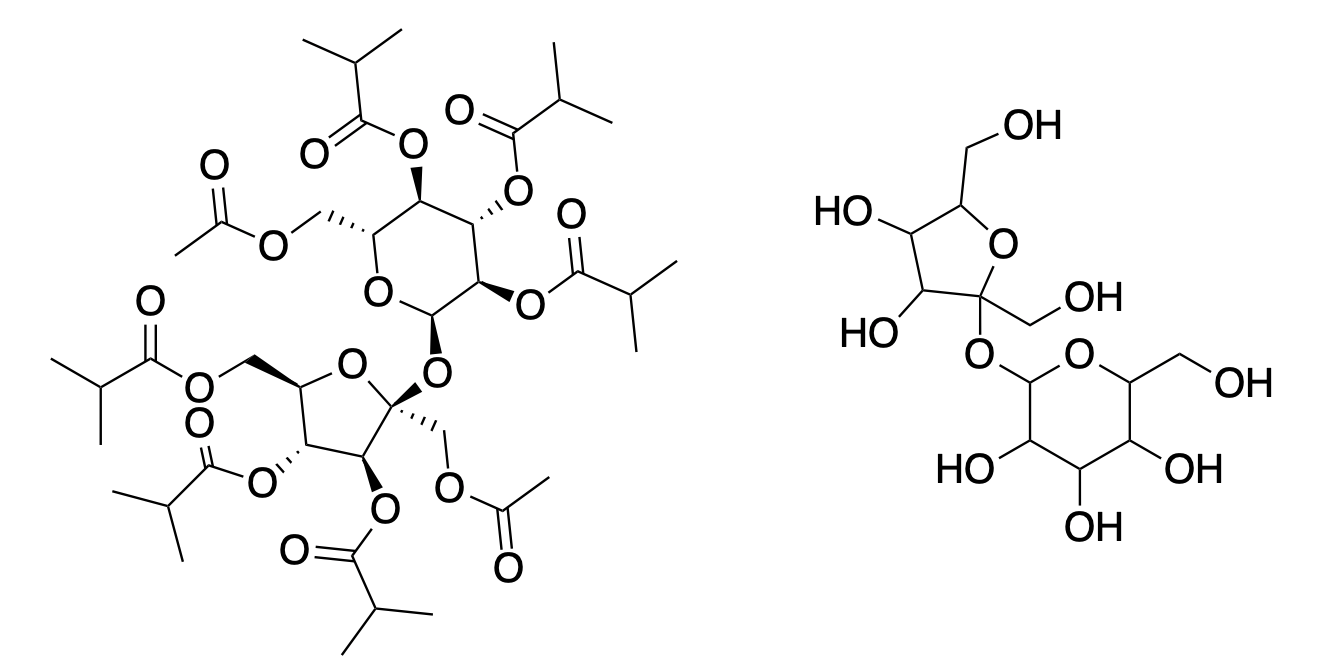
(Left) Sucrose acetate hexaisobutyrate, that pinwheel-looking nightmare, is actually not as crazy as it seems. It is merely sucrose (right), with all of the hydroxyl groups converted to isobutyric esters.
SAH, with a density of 1.16 g/mL, is oil soluble and also heavier than water, making it an effective emulsifying agent.
Bottom line
BVOs were given "generally recognized as safe" (GRAS) status in 1958. Whether harm, if any, they've done since then is anyone's guess, but getting rid of them still makes sense.
NOTES:
(1) In many, if not most, cases, the artificial fruit flavor added to the soda is the identical chemical produced by the fruit.
(2) The Astros cheated by coming up with a clever but illegal method of stealing signs from the opposing catcher to the pitcher, meaning that the batter knew what pitch was coming, a big advantage. MLB penalized the hell out of the team. Unfortunately, they continued to be good, winning the World Series in 2022. They are probably the most hated team in baseball.
(3) Iodine is first.
(4) Solutions are, by definition, clear (note: this does not mean colorless). Suspensions are never clear.



