As if chemistry isn't excruciating enough. You study it for a year or two and, if you're lucky, it makes sense. And then you run into stuff like this:
1. LSD is commonly called "acid." It shouldn't be
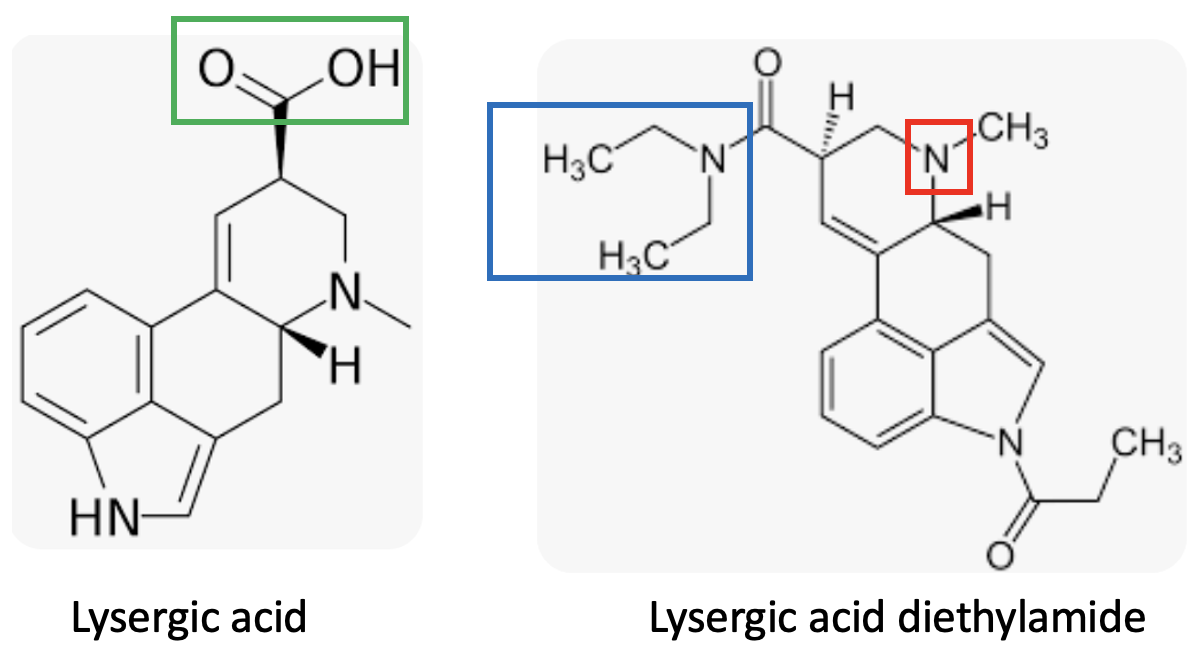
(Left) Lysergic acid has a carboxylic acid group (green box). If it were nicknamed "acid" that would be chemically acceptable, but this is not the hallucinotic drug people use. (Right) But the real drug that is called "acid" is lysergic acid diethylamide. It lacks a carboxylic acid because it has been synthetically modified to convert the carboxylic acid into its corresponding diethylamide, which is neutral. In fact, it's slightly basic because of the tertiary amino group (red box). So LSD should really be called "amide," but the phrase "dropping amide" isn't all that catchy.
ACSH Senior Fellow Dr. Henry Miller has his own theory:
"Maybe the allusion to LSD as an 'acid' originated with someone who had ingested it."
Uh, Henry, how would you know this?
2. Chemistry names can be crazy
There are two chemicals that are pronounced FLOR-ene. But they have absolutely nothing to do with each other other than the pronunciation. Fluorine is the most reactive of all elements and will burn up pretty much everything it comes in contact with. Including a chicken. Fluorene is an aromatic hydrocarbon derived from coal tar, which is mostly inert. Physically, they couldn't be more different.

(Quiz: The name fluorene actually makes sense. Why?)
3. Low-octane gas has no octane. Neither does high-octane.
Once leaded gasoline went bye bye a different anti-knocking chemical was needed. This is where the octane ratings, like 87 and 91 come from. But this is another misnomer.
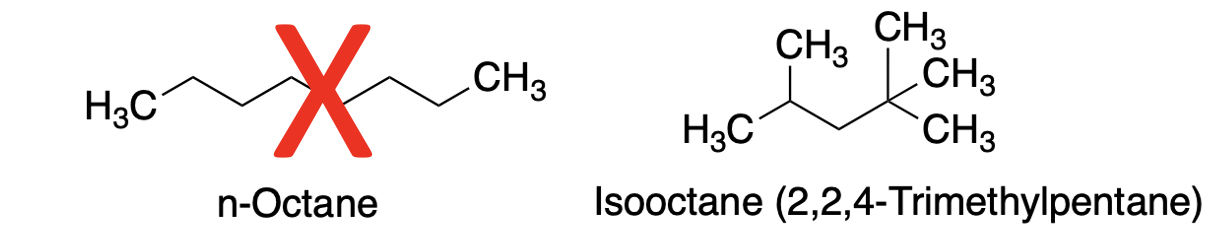
The primary anti-knocking agent in gasoline is called isooctane, aka 2,2,4-trimethylpentane. There is little or no octane in the tank. (1)
4. Chocolate chemicals are crazy too
There is a class of simple, purine-derived (2) stimulants called methylxanthines that are found in a number of plants (Figure 1). The best-known is caffeine, aka, 1,3,7-Trimethylpurine-2,6-dione. Remove a single methyl group (yellow arrow) from caffeine and you have theophylline, aka 1,3-dimethyl-7H-purine-2,6-dione, an old and now little-used asthma drug. Although theophylline has a number of sources, black tea has the most.
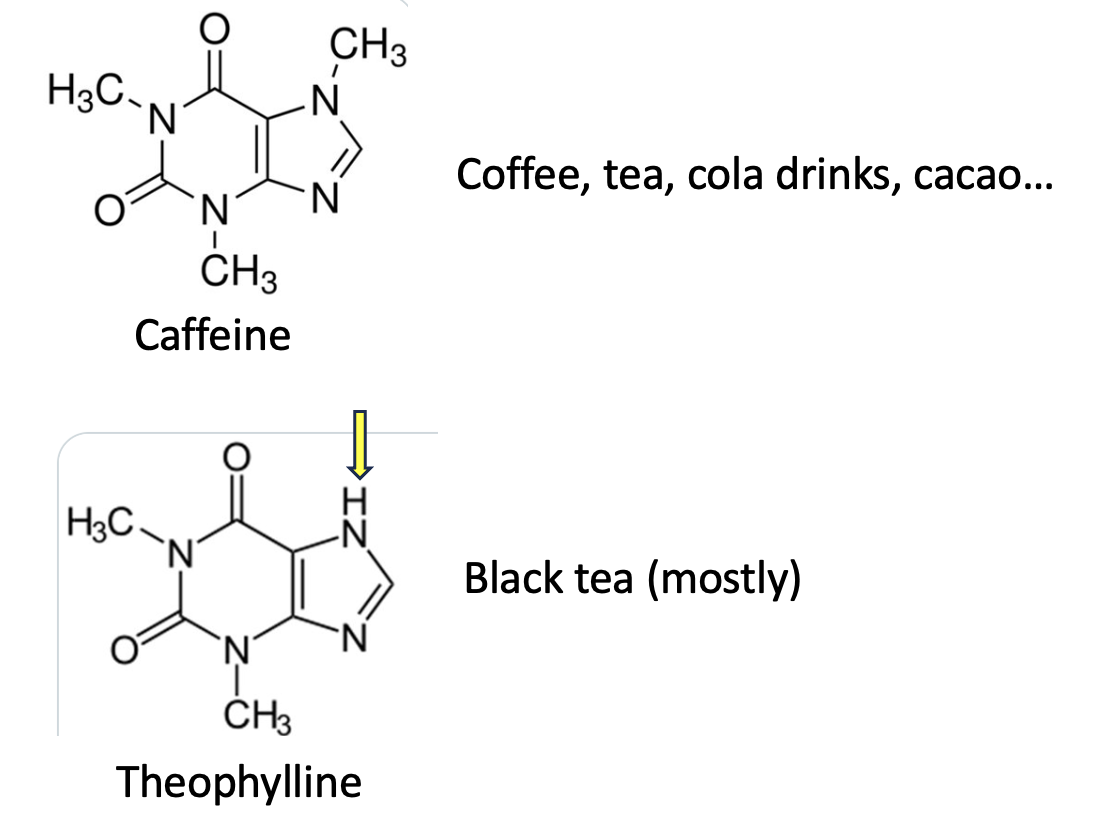
Figure 1. Caffeine and theophylline are chemically similar methylxanthines.
Then there's this:
The primary methylxanthine from cacao beans is called theobromine, aka 3,7-dimethyl-1H-purine-2,6-dione. It is an isomer of theophylline. Below is the structure along with a puzzled bromine atom wondering if it has been the victim of identity theft.

No bromine atom has ever been near theobromine. There's probably some reason for its name but I'm not in the mood to look it up.
5. Pineapple can be made from puke. And the other way around
Most fruit flavors, both natural and artificial, are low molecular weight esters. Esters are made from a carboxylic acid and an alcohol:
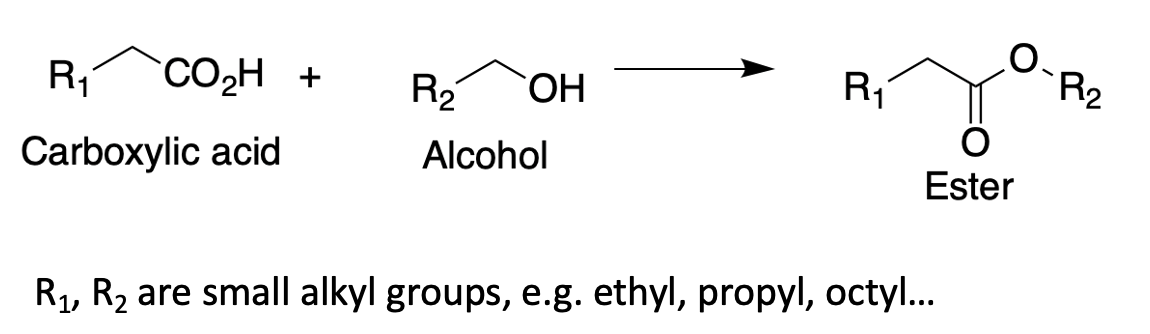
Depending on the selection of R1 and R2 there is (theoretically) an infinite number of esters that nature (of a chemist) can make. In reality, it's about a dozen or two. Here's one:
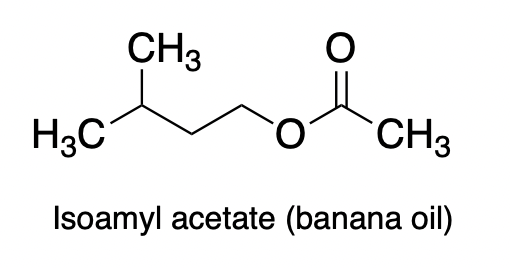
And my favorite:

Yep, that's right. You can convert one of the most vile-smelling chemicals to the same chemical found in pineapples.
Make your own puke!
Esters form under acidic conditions and break down (returning to the same alcohol and carboxylic acid that they were made from) under basic conditions. Which leads to a very dumb idea...
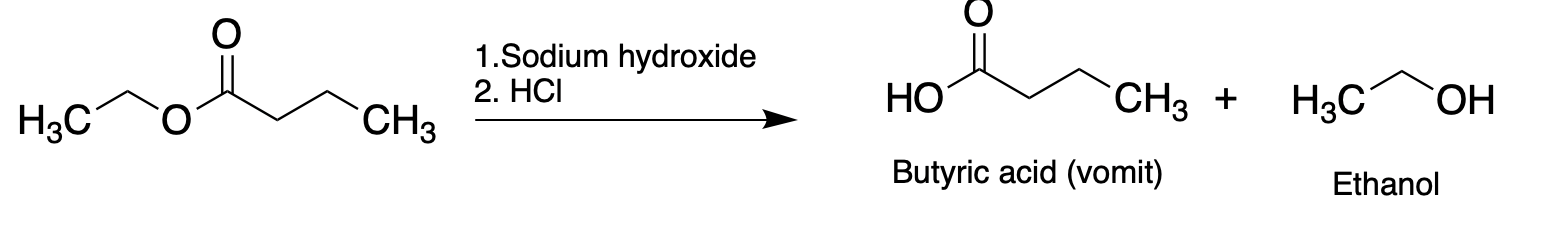
If you take a pineapple product and stir it with dilute sodium hydroxide the ethyl butyrate will quickly break down to its original components. If this gemisch is neutralized with dilute acid and you stuck your nose in it you will not be pleased. It will smell just like vomit and you will have accomplished a miracle: creating the aroma of puke without have being to go to the trouble of puking. If that doesn't belong on the family Christmas card what does?
Not everyone agrees with the significance of this accomplishment. ACSH Research Associate Julie Kasel offers the following:
"You’re really missing out on eating sushi, drinking too much afterward, and puking it up cooked on an East Village sidewalk."
Julie Kasel, ACSH Research Associate
There is only one way she could know this. Perhaps Ms. Kasel will write a book with her own recipes for cooking raw fish without a stove. A lovely image to conclude with, no?
NOTES:
(1) There are various other chemicals used to make up anti-knocking formulas, including ethanol.
(2) Purine is one of the RNA/DNA bases. It is a very common biomolecule.