Last year, I wrote an article about Antibe Therapeutics' potentially revolutionary drug (1), ATB-346 - a derivative of naproxen, shown in Phase 2 trials in Canada to reduce pain and inflammation, sparing the stomachs of the participants. The drug is now called otenaproxesul and the company got thumbs up from the FDA, which just approved its IND (investigational new drug) application.
An IND is the first of many hurdles that a potential drug must face on its way to the pharmacy (1). It gives the company permission to conduct first-in-man experiments.
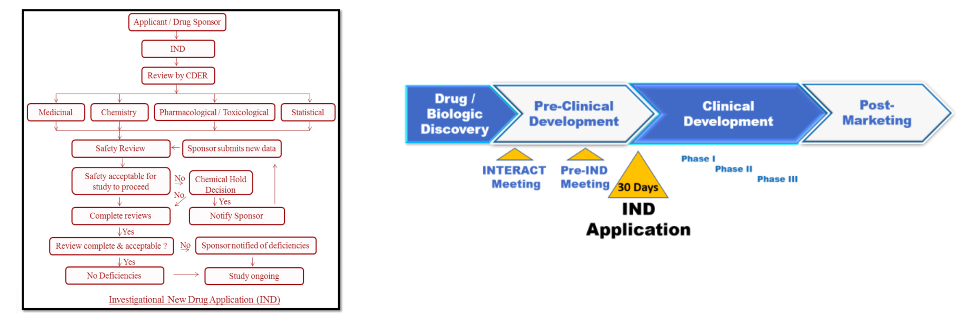
(Left) A flow chart of what is required to file an IND. Don't try to read this. You will have a nervous breakdown. (Right) An IND application is the first step in evaluating the safety of a new drug in humans. Diagrams: International Journal of Regulatory Affairs, Edge
In a sense, Antibe got a "break" from the FDA, not because the company cut corners, but because the agency allowed the company to use Phase 2 data from Canada, which showed that otenaproxesul had a very good safety profile, especially compared to naproxen.

(Left) Phase 2 double-blinded data from Canada showed that otenaproxesul caused far fewer gastric ulcers than naproxen. (Right) The chemical structures of otenaproxesul (L) and naproxen (R). Source: Antibe Therapeutics
Because we completed Phase II trials prior to the IND application, we were able to provide a comprehensive package of preclinical and clinical data in a submission comprising more than 55,000 pages. We look forward to working with the FDA as we pursue development of a gastrointestinal-protective, non-addictive analgesic for the many millions of patients seeking better medicines for osteoarthritis.”
Dr. Joseph Stauffer, Antibe’s Chief Medical Officer 3/29/21
Antibe is following a rather strange path in its development of otenaproxesul. Typically, drug companies initiate clinical trials in the US – the biggest market for prescription drugs. But in this case, Antibe is gearing up to run Phase 3 trials in Canada before even the most preliminary toxicology studies are run in humans. But in this case, where a novel class of an analgesic/anti-inflammatory drug is sorely needed, whatever gets them there fastest is just fine.
NOTE:
(1) Antibe calls its technology a "hydrogen sulfide platform." During my interview with CSO Dr. John Wallace, he explains this technology.
Statement of Conflict of Interest: My IRA contains some Antibe stock. Easily enough to buy a hot dog at Yankee Stadium. Maybe even two of them.




