
In March I wrote about a novel NSAID-like drug being developed by Antibe, a Toronto-based drug company. The drug, which is called ATB-346 (now called otenaproxesul) is structurally and functionally similar to naproxen (Aleve) but without its gastrointestinal side effects, especially ulcers (see Pain Relief And No Ulcers? 'Magic Aleve' In Clinical Trials).
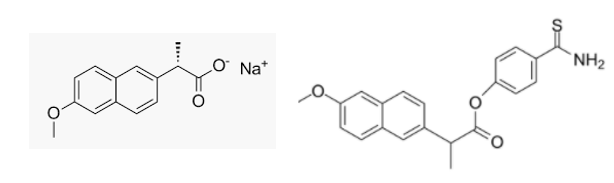
The chemical structures of naproxen sodium (L) and otenaproxesul (R).
The company just issued a corporate update saying that "Human Proof-of-Concept [is] Now Firmly Established for ATB-346." That's a pretty significant statement because if it holds up in Phase 3, otenaproxesul would be the first of a novel class of analgesics. This is no small accomplishment since the other two, NSAIDS and opioids have been the only choices for 125 years. Keeping in mind that the success rate going from Phase II to Phase III is less than 31% (and this is a corporate announcement, not a published paper), let's take a look at the company's claims. In my March article efficacy of otenaproxesul (Phase IIa) was determined in a very small group of patients.
- Depending on the dose, patients who took otenaproxesul experienced a 39-44% improvement in the WOMAC score of pain (the primary endpoint) after two weeks.
- WOMAC scores of stiffness and difficulty in performing daily activities (secondary endpoints) were also significantly improved.
- Unlike Phase IIa studies, which enrolled only 12 patients, the Phase IIb study, which was randomized, blinded, and placebo-controlled had 360 patients.
- According to Antibe, NSAIDs usually reach maximum effectiveness after 6-8 weeks; otenaproxesul showed significant improvement in two weeks, so it is not unreasonable to expect additional efficacy upon longer exposure (speculation only).
- (I don't quite get this next part.) It is well-known that NSAIDs work by inhibiting the enzyme cyclooxygenase (COX) (1). Otenaproxesul, according to the statement, inhibited >90% of COX enzymes at all doses, yet was essentially devoid of GI side effects (2).
- NSAIDs increase blood pressure which is considered to be a contributor to the cardiovascular risk of these drugs. But according to Antibe: "Trial participants treated with ATB-346 experienced neither an increase nor decrease in blood pressure in contrast with other NSAIDs, which often increase blood pressure. Blood pressure increases are viewed by medical practitioners globally as being an important proxy for the cardiovascular risk of NSAIDs. The absence of an increase in blood pressure has been a consistent finding in all of ATB-346’s clinical trials to-date and suggests a favourable cardiovascular safety profile for the drug."
Bottom line:
- Otenaproxesul continues to look promising, especially its safety profile. What about potency? Will it work well enough? This will be established in Phase III trials. Even if the drug is equipotent to naproxen (Antibe CSO Dr. John Wallace claims that it is 6-fold more potent than naproxen. You can read my interview with Wallace here), based on its safety profile the drug should be able to be taken at higher doses and for longer periods of time.
- It is important to remember that the announcement was a corporate update for shareholders. Take that for what it's worth. If the report is overly optimistic it wouldn't be the first time.
- Let's hope that Phase III data look as good or better. If otenaproxesul is approved this would represent an important (and much needed) advance in the treatment of pain and arthritis (3).
(1) Cyclooxygenases are enzymes that are responsible for the conversion of arachidonic acid to prostaglandins, extraordinarily powerful hormones that control a wide variety of processes including pain and inflammation.
(2) This may be due to selective inhibition of COX-2 which is how Celebrex works. Standard NSAIDs inhibit both COX-1 and COX-2, both of which play a part in pain and inflammation. But inhibition of COX-1 also decreases the protective lining in the stomach, which is thought to be responsible for ulcers, etc. But it's not that simple. Inhibition of COX-2 may also cause significant GI side effects.
(3) Having been officially (and unanimously) accepted into "The Old Fart" club I have a vested interest in the success of this drug. You try 9 innings of fast-pitch softball at my age. My hair hurts afterward.
Disclaimer: My IRA contains a very small (less than 1%) amount of Antibe stock
Disclaimer #2: If you think that's why I'm writing this article you're out of your mind.



