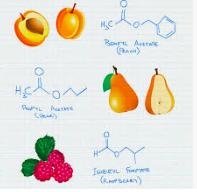
Organic chemicals can be divided into broad categories – let's call them "flavors" – based on what functional groups they possess. A simple way to look at this is that chains or rings of carbon atoms make up the framework of a molecule and the functional groups supply the decoration, which gives it its properties like chemical reactivity, solubility, toxicity, and odor. For example...
Start with a simple hydrocarbon, let's say ethane. It has no "flavor" or odor. It is "chemical white bread."Now, let's replace one of the hydrogen atoms on ethane with a variety of common atoms (or groups of atoms) called functional groups. (Figure 1 below - replacement of one ethane hydrogen with common functional groups). This seemingly small change results in a striking difference.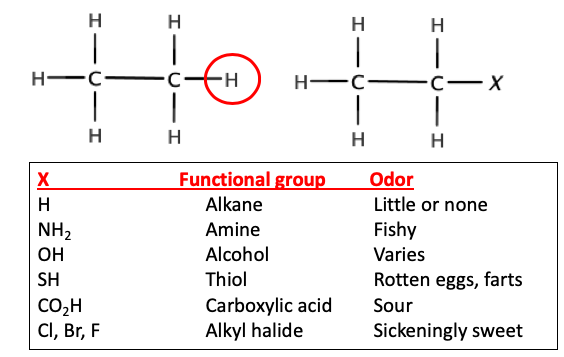
Figure 1. Examples of common functional groups.
This CHEMISTRY LESSON FROM HELL® is going to focus on esters – a large class of chemicals, both naturally occurring and synthetic, which have two things in common:
- They smell good (1)
- Their two components – alcohols and carboxylic acids – don't (especially carboxylic acids - blah!)
An ester is formed when an alcohol and carboxylic acids are combined, eliminating a molecule of water in the process. Here's a generic reaction:
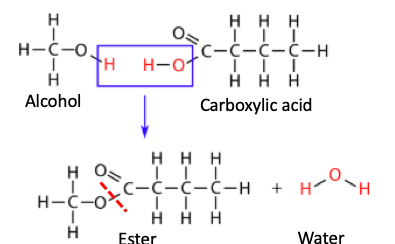
An esterification reaction: An alcohol reacts with a carboxylic acid forming an ester (new bond is indicated by red hatch line) and water. This reaction is called the Fischer esterification and it's 125 years old. Original image: Libre Texts
So, the chemical definition of an ester is a molecule formed by the combination of an alcohol and a carboxylic acid:
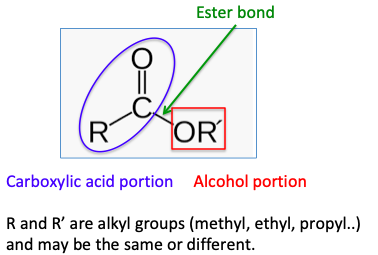
What's especially intriguing about esters is that even when there are only small differences in the number of carbon atoms in either the carboxylic acid part of the ester or the alcohol part the difference in the esters can be significant. Here are two examples:
1. Acetic acid is held constant the "acid part" of the molecule and it is combined with six different alcohols to make six different esters.
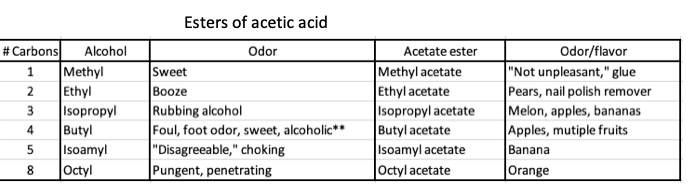
Esters of acetic acid. Note the differences in the ester when it is combined with six different alcohols.
** I cannot come up with any explanation for butyl alcohol being described in such different ways. Crazy.
2. Ethyl alcohol, held constant as the alcohol component, is combined with five different carboxylic acids.
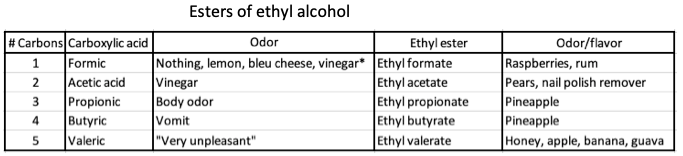
Esters of ethyl alcohol combined with five different carboxylic acids. Note that the carboxylic acids are nasty smelling, but their ethyl esters are anything but.
*The different descriptions of formic acid seem insane but there is a genetic component behind this.
Why do esters smell so nice? There could be a number of reasons. There is a genetic advantage for plants to have their fruit eaten; it spreads the seeds allowing new plants to grow. Likewise, animals, birds, and insects may have co-evolved to learn that sweet smells and tastes mean that good (and safe – esters are not toxic at the levels found in foods) food is available. Another reason may be that low molecular weight alcohols and carboxylic acids are readily biosynthesized by plants. Or some combination of all of these.
Let's finish with...
Stupid chemistry time! (Image: TeeSpy)

When nature isn't natural.
One of the properties of esters I haven't mentioned is that they are volatile, sometimes even more so than either of their components. And this is despite the fact that the molecular weight of the ester is (necessarily) higher than either of its components (Volatility is, in general, inversely proportionate to molecular weight). (There are chemical reasons for this but I don't want my readers on Amazon looking for cyanide, so I'll skip it.) For example, consider the boiling points of two esters and their component alcohols and carboxylic acids:
Alcohol Carboxylic acid Ester
Ethyl alcohol - 78ºC Acetic acid - 118 ºC Ethyl acetate 88ºC
Butyl alcohol - 118ºC Butyric acid - 164ºC Butyl butyrate - 165ºC
For a plant that "wishes" to attract predators ester volatility is a good property. But in the food business not so much. Or the science business either. Let's say that you are a manufacturer canning fruit that contains methyl propionate (fruity, rum-like scent). But methyl propionate's boiling point (80ºC) is so low that some of it evaporates during the process. The result - the flavor isn't so great, so you decide to add a little of the exact same chemical to bring the flavor/scent back to where it was before canning. But now, the methyl propionate that was added back is considered to be an artificial flavor, despite it's identical in every way to the methyl propionate that evaporated. But it must now be labeled "artificial." This stupidity is one of the many ways that the organic food industry pulls the wool over our eyes. Your body has NO idea whether the canned peaches you are eating have the methyl propionate from the plant or some of it replaced from a bottle. Methyl propionate is methyl propionate, no matter where you get it.

I hope you enjoyed the chemistry lesson on esters. But if you (like 99% of the victims readers fell asleep, I wish you sweet (smelling) dreams.
NOTE:
(1) This assumes that the ester is volatile enough to get into your nose. Many are not, especially when the molecular weight is high.