To project military, cultural, and economic power, the ancient Romans relied on a technology that we often take for granted today: roads. A spiderweb of highways allowed soldiers and goods to travel quickly from one corner of Europe to the other (and beyond). Hordes of “barbarians,” however, exploited those veins and arteries of the empire to sweep across the Mediterranean and ultimately sack Rome in 410 AD.
Just as the Roman roads helped the Visigoths run roughshod over Southern Europe, cancer’s invasion of distant organs exploits literal veins and arteries. (How’s that for a segue?) It’s fitting that the fifth (and final) installment in my series covering the “war on cancer” discusses such a military-sounding topic. But rather than take a soldierly perspective, I’ll adopt a molecular one. We’ve already discussed cancer’s proclivities for genetic mutation and drug resistance, but arguably its most dangerous behavior is its knack for colonizing other parts of patients’ bodies.
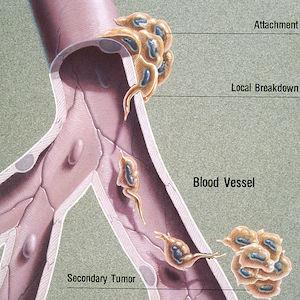
The complex—and still mostly unknown—process by which cancer spreads is called metastasis. Many tumors are continuously shedding cells that can infiltrate and navigate the bloodstream and lymphatic system, which function as the body’s highways. The new tumors that form (called metastases) resemble and behave like the “primary” tumor they came from; for example, a melanoma that has metastasized to the brain is abnormal skin tissue, not abnormal brain tissue. In some cases, an oncologist will detect metastases but won’t be able to determine the location or type of the primary tumor. This rare condition is called carcinoma of unknown primary (CUP).
Most cancers that form solid tumors will metastasize if given enough time, at which point the patient’s prognosis nosedives. Metastatic disease is typically characterized as the most advanced stage of cancer (stage IV), and it has been estimated to account for roughly 90% of cancer deaths. Current treatments may be able to slow disease progression and alleviate symptoms, but they are rarely curative. Certain types of metastatic cancer, however, are much more receptive to therapy—just ask Lance Armstrong about the testicular cancer that had spread to his lungs and brain.
A common theme of this series has been that cancers often behave differently from one another—they are different diseases, after all—so it should be no surprise that some cancer types are more likely to metastasize than others. The tendency can vary even between cancers from the same tissue. Melanoma, for example, is so dangerous because it is highly prone to form metastases in the brain, lungs, bones, and other locations. However, other skin cancers like basal and squamous cell carcinomas rarely spread and are therefore much less serious (though it’s still important to deal with them as quickly as possible).
Similarly, not all organs are equally receptive to pioneering cancer cells. Cancers commonly spread to the bones, liver, and lungs, but each cancer type also has its own set of preferred locations for metastases to form. This phenomenon has led to the “seed and soil” hypothesis of cancer metastasis. Just as the seeds of a plant can only take root and thrive in a patch of soil with the right nutrients, water content, microbes, etc., circulating cancer cells must find a suitable landing spot among the body’s various organs and tissues.
Cancer research has tended to focus on the seeds at the expense of the soil, but it’s impossible to get a full picture of what’s going on if they’re not considered simultaneously. Even though I mentioned above that metastases tend to resemble their primary tumors, tumors growing in separate “microenvironments” tend to behave differently from one another and may not respond to the same treatments. When you also consider exponential growth, tumor heterogeneity, and other fundamental aspects of cancer biology, it becomes clear how metastatic cancer can quickly get out of hand and overrun our bodies’ defenses.
One of the main barriers to treating metastatic cancer is the lack of effective methods for studying it in the lab. Much of cancer research is performed by studying cells in flasks, but it’s likely challenging—if not impossible—to properly simulate the dynamics of metastasis in a dish. And while mice are often used as living, breathing models of humans with cancer, mice aren’t people, and they certainly don’t have our immune systems, bloodstreams, or lymphatic systems.
To start to solve this problem, let’s take a page from the Romans’ playbook and emphasize the simple, unsexy essentials that often get overlooked. A new sword may be flashy, but well-functioning roads and aqueducts can turn a warlord into an emperor. We need to invest in basic research so we can learn more about our enemy and figure out what we’re aiming at.
Let’s hit singles and doubles—swings for the fences yield too many whiffs. Let’s eschew political grandstanding—cancer doesn’t care about empty campaign promises. And most importantly, let’s follow the path that the data have outlined—it may not give us everything we want, but it will take us in the right direction.