The good news? There's a very effective antiviral drug called Paxlovid that will keep you alive and out of the hospital when/if omicron gets you, and a second drug, molnupiravir, which doesn't work as well, but can still be helpful. The bad news?
Drugs & Pharmaceuticals
Following the FDA's granting of Emergency Use Authorization to Merck's molnupiravir and Pfizer's Paxlovid, the only two approved direct-acting antiviral drugs; we now have two badly needed tools to deal with COVID, especially since it is
Today, NBC News managed to spoil the coming-out party of a desperately needed, lifesaving drug with its lead headline of the morning:
"Pfizer antiviral pills may be risky with other medications."
It's not totally absurd to compare our war against COVID with a boxing match. The virus clearly won Round 1; aside from masks and isolation, we were pretty much defenseless.
To all those who are skeptical because they worry that a government-sponsored investment in the broken antibiotic marketplace is a give-away to the pharmaceutical industry, I have one word for you – covid!
OK, that last line is a bit of clickbait; placebos themselves are not necessarily becoming more effective, but newer active medications may not be much more effective than the older ones, which means we might need to reconsider how we determine ac
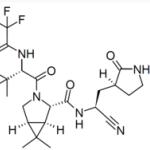
Recent rumors speculate that ivermectin and Pfizer's promising experimental Covid drug PF-07321332 (1) are both inhibitors of the viral main protease (Mpro) (2) and can therefore be used
In a sense, we are fortunate. That is if you can call 100,000 overdose deaths per year, mostly due to fentanyl, fortunate.
Medicare is running a pilot program capping the monthly cost of insulin, but there is no federal regulation in sight.
The "COVID era" is reteaching us a lesson that was learned in the 1990s and 2010s – that an effective direct-acting antiviral (DAA) medication can work wonders in controlling or eliminating a serious viral infection.