If you've ever worked in a chemistry lab, you already know the answer: No. Or maybe yes. It depends on what you're looking to accomplish.
While the chemistry reported in a new Science paper is rather clever, the title "Low-temperature mineralization of perfluoro carboxylic acids" suggests that you could stick PFAS in a beer cooler, whistle the National Anthem, and bye-bye chemicals. Not even close.
William Dichtel, a professor in the Department of Chemistry at Northwestern University, and colleagues devised an ingenious method for destroying PFAS – a class of chemicals that had all their hydrogen atoms replaced by fluorine. This chemistry isn't trivial because of the properties of PFAS. In particular, the very strong carbon-fluorine bond that makes perfluoro chemicals useful for products like non-stick cookware also makes them hang around forever because they are chemically inert. Unlike most chemicals, PFAS do not degrade, either metabolically or environmentally; they accumulate. So, minuscule quantities of these chemicals can be found pretty much everywhere, albeit at extraordinarily low concentrations.
The potential impact of PFAS on human health is currently a very hot and controversial topic. Studies have shown an association between the presence of PFAS in people and various health conditions. There is no need for me to delve into this. ACSH advisor Susan Goldfaber has done an excellent job discussing the science of health claims and PFAS. She concludes that most of them are nonsense. See PFAS: Fear And Misinformation Runs Wild.
The chemistry in the Science paper is both simple and complex. It discusses one set of conditions degrading perfluoro molecules in a repetitive, stepwise process (Figure 1). (Those who fear complex reaction sequences should leave now.) The simplicity comes from using a single reagent to perform all the reactions required to break down the PFAS. More on this later.
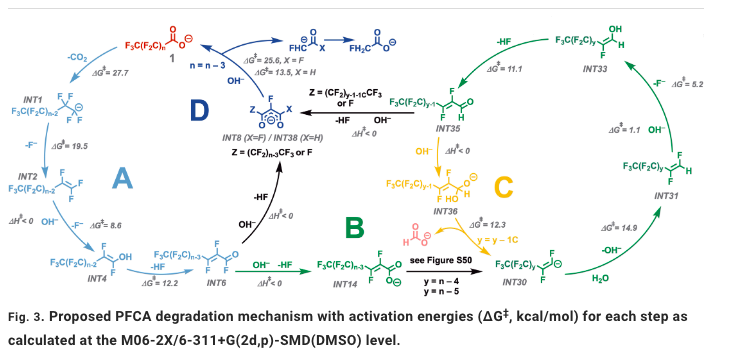
Figure 1. Proposed mechanism for the stepwise breakdown of perfluoroalkyl carboxylic acids into smaller fragments. Don't say I didn't warn you Source: SCIENCE, 18 Aug 2022, Vol 377, Issue 6608, pp. 839-845, DOI: 10.1126/science.abm8868
(Note to readers: Despite not being a professional chemist for 12 years, I actually understand the above monstrosity. I also understand that if I try to explain it, I will (and rightfully so) be getting enough anthrax-filled envelopes to fill a U-Haul, so save the postage.)
But, once a chemist, always a chemist. Which makes the following apology a requirement.
It's time for yet another episode of...

Your hosts of TDCLFH® Steve and Irving return! If you love fried eggs then hell is a pretty tasty place to be! Steve (left) never starts a day without hot coffee (iced coffee is a rare commodity), a couple over easy and fried bacon, crispy, of course. But Irving started experimenting with veganism. It is not going well. His Amazon Fresh Direct box of organic kale arrived only to burst into flames immediately upon removal from the box. Looked like someone smoked a few cartons of Marlboros, but the taste was unchanged.
The chemistry is elegant in its simplicity. It involves using a base plus heat to perform some difficult transformations. Here they are (Figure 2). Look away if you must.
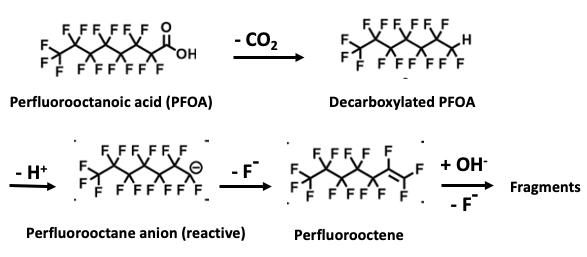
Figure 2. Systematic degradation of PFOA by base and heat. 1) Perfluorooctanoic acid (PFOA), a member of the PFAS class, loses carbon dioxide (the carboxylic acid is replaced by a hydrogen atom) - a common reaction called decarboxylation. 2) The hydrogen is removed (as a proton) forming a short-lived, reactive anionic intermediate. 3) The anion loses fluoride to form an alkene (in this case, perfluorooctene). 4) An addition-elimination reaction continues the process, eventually breaking down the PFAS. No, I'm not going to describe this.
Are these conditions really "mild?"
Compared to a nuclear reactor in the middle of a meltdown, yes. But the conditions required are sodium hydroxide dissolved in dimethyl sulfoxide (DMSO) with a little water, which is heated to 120oC (248oF). DMSO and sodium hydroxide make a very strong base, which will melt not only your face but also the face of the guy in the next lab. Here's the equation:
NaOH + DMSO + Lots of heat + Face --------> No Face + Soap
Your face will have undergone a process called saponification – the breakdown of fats by lye to form soap, a reaction that has been around since 2800 BC.
Is this practical?
If your goal is to remove trace amounts of PFAS from water, of course not. There is no way to simulate these conditions in a water purification facility. But this paper is not about removing PFAS from the environment; it is a very good study of the conditions and mechanism of a new method of PFAS degradation reaction that could be used to destroy large quantities of pure PFAS instead of existing methods, for example, incineration or high-energy oxidation.
There are already a variety of methods that can be used to treat drinking water. A 2019 article in C&E News explains these very nicely.
For this and other must-know science news and analysis you should, of course, stick with ACSH.




