An article in The People's Daily Online, China's largest newspaper, reports that Azvudine (aka FNC), a reverse transcriptase inhibitor (1) – is being approved as an inexpensive treatment for COVID-19. The paper reports a dramatic improvement in patients who were given the drug, which was "homegrown," developed by Genuine Biotech Company, which is based in Henan province. The drug has been hailed as a readily available, inexpensive alternative to Paxlovid.The People's Daily Online
[T]he medication's price has not been determined, but should be much lower than pharmaceutical company Pfizer's Paxlovid pill, which costs about 2,300 yuan ($340) per dose.
Chang Junbiao, a leading researcher of the pill and the vice-president of Zhengzhou University in People's Daily Online
The Big Question - Do We Believe It?
Since I've been following the progression of COVID antiviral research for well more than two years, I can say with absolutely no doubt that a second effective COVID drug, especially one that operates by a mechanism that is different from that of Paxlovid (3), would represent a huge advance in the endless battle against an ever-changing virus.
At least upon first glance, Azvudine seems to work. According to Reuters:
In a late-stage clinical trial, 40.4% of patients taking Azvudine showed improvement in symptoms seven days after first taking the drug, compared with 10.9% in the control group, Henan province-based Genuine Biotech said in a statement earlier this month..."
While this sounds mighty impressive you should withhold judgement until you read the rest of it:
...without providing detailed readings.
Translation: no data (2). Rather than wade into global health policies or whether China, not exactly known for its free press, is telling the truth, the absence of any data automatically calls into question the veracity of this statement. The closest thing to "data" I can find is encouraging, but it isn't really data:
“[In] the trials … the participants’ nucleic acid tests turned negative in about three to four days... [The results suggest use of the] drug can be stopped in six to seven days and patients can be discharged from hospital in nine days.”
— Jiang Jiandong, the director of the Institute of Medicinal Biotechnology. Presented at the Chinese Academy of Medical Sciences, February 2022.
Some Science
Unsurprisingly, the chemical structures of azvudine and AZT are quite similar (Figure 1).
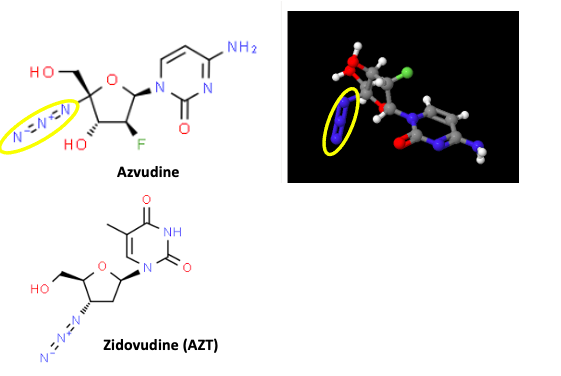
Figure 1. (Top) Azvudine is shown in its two-dimensional (standard) structure on the left. On the right is a 3D model. The azido groups are highlighted by yellow ovals for clarity. (Bottom) Zidovudine: The name is similar as is the structure, but this drug is AZT, the first approved but ultimately doomed HIV/AIDS drug in the US.
Is it possible that Azvudine is the second magic bullet that might help us finally get COVID under control? There are some obvious questions:
- The drug was developed in 2014 as a potent inhibitor of an HIV-1 mutant. HIV is a retrovirus; SARS-CoV-2 is not, although there is no reason that a drug that inhibits HIV by one mechanism cannot inhibit SARS-CoV-2 by a different (but similar) mechanism. They are both RNA viruses.
- Potency concerns me. Here's why:
Below are reported IC50 values – the concentration at which the viral replication is decreased by 50% in cultured cells (4) of four known SARS-CoV-2 inhibitors. The lower the number, the more potent the inhibitor; therefore, less of the drug needs to get into the blood to inhibit viral replication. It is important to keep in mind that in-vitro potency values can vary widely depending on the conditions employed, the type of cells used, the way the value is measured, and what lab is running the experiment. So, it is more accurate to evaluate the following data as an approximate measure of the drug's strength. Nonetheless, some things become clear.
Paxlovid ~ 0.02-0.2 micromolar (~20-200 nanomolar (nM))
Molnupiravir ~ 0.14-1.77 micromolar (140-1,770 nM) – roughly 10X less potent than Paxlovid
Remdesivir ~ 0.77-1.65 micromolar (770-1,650 nM) – roughly 12X less potent than Paxlovid
Azvudine ~ 1.2 and 4.3 micromolar (1,200-4,300 nM) – roughly 28X – less potent than Paxlovid
It is not a coincidence that Paxlovid is currently the standard of care for treating COVID; it is significantly more potent than molnupiravir, remdesivir, or Azvudine. To put all of this in perspective, here's a chart that shows (qualitatively, not quantitatively) how potency is an important determinant (one of many) of a successful drug. Specifically, here is the four Covid drugs stack up in potency and (roughly) how this may translate into utility as a drug (5).

**Note that Azvudine is a highly potent (sub-nanometer) inhibitor of HIV but its potency against SARS-CoV-2 is about 1000-times less. Based on these data, it is not clear how the drug can be so effective in Covid patients at a tolerable dose.
Bottom Line
Although the world desperately needs a Paxlovid alternative (if not now, soon enough), there are too many unanswered questions to convince me that this drug will be all that is claimed. I hope I'm wrong, but the drug doesn't appear to be potent enough to produce the kind of results that were reported by the People's Daily Online. Until I see some actual clinical data, I'm simply going to wish them well.
NOTES:
(1) Science Direct provides a nice summary of reverse transcriptase inhibitors.
(2) This is not to say that nothing has ever been published on the drug and Covid. A 2021 paper in Nature reported in which Jiang Jiandong was one of the lead authors reported the compassionate use of Azvudine for a group of 31 Covid patients, not the 1,100 described in The People's Daily Online.
(3) I have explained why "hitting" a virus with two drugs that operate by different mechanisms is a very effective way to minimize resistance.
(4) Depending upon the disease or infection, cultured cell experiments can run the gamut of almost useless to highly predictive of a drug's success. Antiviral research is much closer to the latter.
(5) There are many other factors that determine whether a drug that is potent in a cell-based assay will make it to the drug store. These include toxicity, mutagenicity, metabolism, absorption, and distribution. Drug discovery is anything but easy.




