
Last year, my wife and I moved out of Seattle into a house in the suburbs. One of the many new responsibilities we acquired in the process is taking care of a yard.
As a trained microbiologist, I haven't the foggiest clue about plants or gardening. So I found a nifty little app called PlantSnap, which uses a database of photographs to identify plants1. Using the app, I identified one of the plants in my yard as Sambucus nigra, also known as elderberry. (Specifically, it's a variety known as Black Lace.) It looks and sounds like a plant that could be poisonous, so I looked it up. My instinct was correct2. It's poisonous.
Apparently not everyone is clever enough to spend a few minutes on Google, particularly before consuming materials from exotic plants. Recently, the New York Post reported that a professor at Columbia University who is "a great believer in natural this and that" accidentally poisoned herself after she decided to "take tincture of elderberry instead of a flu shot." It was then that she discovered, "It turns out they have cyanide.”
How a Tincture of Elderberry Turned into a Tincture of Cyanide
It's not quite accurate to say that elderberry contains cyanide. Instead, elderberry -- along with several other plants, including almonds and cassava -- contain what are known as cyanogenic glycosides. That's an organic chemist's way of saying "sugar derivatives that can generate cyanide." How do they work?
Let's use cassava as an example. This plant contains a cyanogenic glycoside called linamarin. The textbook Ensuring Global Food Safety depicts how it breaks down to form cyanide.
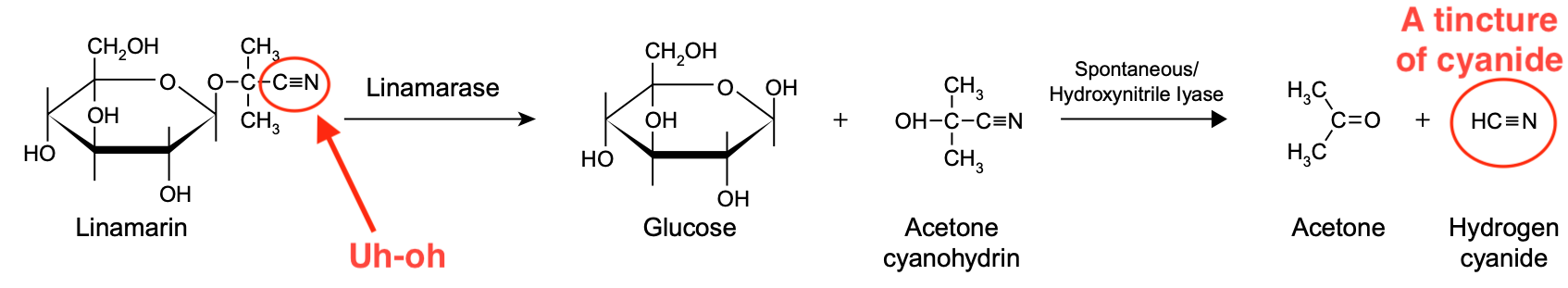
As shown, linamarin is broken down in a two-step process. First, the acetone cyanohydrin group is removed, leaving behind glucose. Second, acetone cyanohydrin is split, yielding acetone and hydrogen cyanide. This is the general pattern for the metabolism of cyanogenic glycosides.
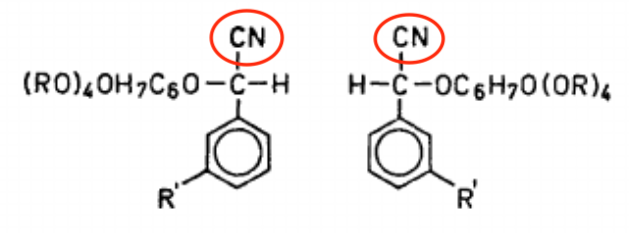 Elderberry doesn't contain linamarin but other kinds of cyanogenic glycosides. (See figure. Source: Acta Chemica Scandinavica.) Once again, notice that suspicious CN (cyano) group. That's what eventually turns into cyanide.
Elderberry doesn't contain linamarin but other kinds of cyanogenic glycosides. (See figure. Source: Acta Chemica Scandinavica.) Once again, notice that suspicious CN (cyano) group. That's what eventually turns into cyanide.
Why exactly a professor would believe that a "tincture of elderberry" could serve as a substitute for an influenza vaccine remains a mystery. Let's hope she at least learned a science lesson.
Notes
(1) The app doesn't work as well as I'd hoped. The same plant can receive wildly different identifications depending on which part is photographed and at which angle the photo is taken. But it works well enough for amateurs like me.
(2) To be fair, I think every plant is poisonous, especially vegetables.



