For the first time ever, the Food and Drug Administration approved a mobile app to help treat substance abuse disorders.
On Thursday, the agency gave the green light to the tech firm Pear Therapeutics, which developed reSet, a digital device that delivers therapy assistance to those suffering from cocaine, marijuana, alcohol or stimulant abuse. It is not intended to be a standalone approach, but rather used in conjunction with outpatient addiction therapy presently at the heart of standard substance abuse treatment.
“This is an example of how innovative digital technologies can help provide patients access to additional tools during their treatment,” said Carlos Peña, Ph.D., M.S., director of the Division of Neurological and Physical Medicine Devices, in FDA’s Center for Devices and Radiological Health, in a statement. “More therapy tools means a greater potential to help improve outcomes, including abstinence, for patients with substance use disorder.” In the process, the agency created a new product group of low-risk "digital therapeutics."
In explaining the scope of its approval, the FDA pointed out that the app was not approved to treat opioid dependence disorders, as the "clinical trial did not demonstrate the effectiveness of using the Reset device in patients reporting opioids as their substance of abuse."
ReSet, which can only be prescribed, delivers a brand of talk therapy known as Cognitive Behavioral Therapy, or CBT, which is recognized as an effective treatment for those with substance use disorders, or SUD. And to encourage its use outside the sphere of clinicians, the app utilizes a series of awards for patients to stay clean and to stick with the program. Therapists monitor a patient's progress online by utilizing a clinician dashboard on a desktop computer.
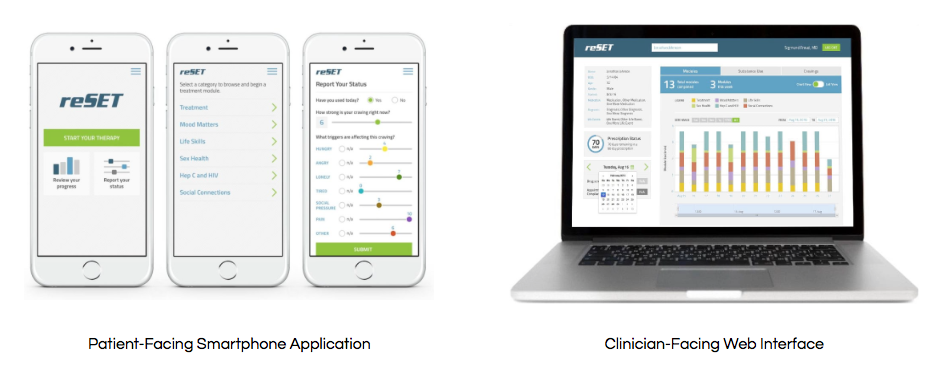 Pear conducted a three-month clinical trial involving nearly 400 patients – who either utilized standard treatment, or the standard treatment along with the desktop-version of reSet – and the FDA found the findings to be convincing.
Pear conducted a three-month clinical trial involving nearly 400 patients – who either utilized standard treatment, or the standard treatment along with the desktop-version of reSet – and the FDA found the findings to be convincing.
"The data showed a statistically significant increase in adherence to abstinence for the patients with alcohol, cocaine, marijuana and stimulant SUD in those who used Reset, 40.3 percent, compared to the patients who did not, 17.6 percent," the agency wrote.
For its part, with the FDA's stamp of approval Pear Therapeutics sees itself on the forefront of future treatment advances.
“This is a defining moment for digital therapeutics and for patients with substance use disorder,” said Corey McCann, the company's President and CEO, in a statement. "We believe that prescription digital therapeutics hold promise in improving patient outcomes across a wide range of central nervous system disorders including psychiatry, neurology and pain, and will become a vital part of tomorrow’s treatment paradigm across all disease areas."




