Dr. Cox: Did you actually just page me to find out how much Tylenol to give to Mrs. Lendsner?
J.D.: I was worried that it could exacerbate the patient's...
Dr. Cox: It's regular strength Tylenol. Here's what-chya do: Get her to open her mouth, take a handful, and throw it at her. Whatever sticks, that's the correct dosage.
Of Dr. Cox's many rants on the comedy show Scrubs, this is definitely one of the funnier and more memorable1. However, it isn't quite accurate.
Tylenol (a.k.a. acetaminophen or paracetamol2), unbeknownst to many, is actually a fairly toxic drug. Its ubiquity has lulled us into a false sense of security about its safety. But as our resident chemist Dr. Josh Bloom expertly explained, Tylenol "has a low therapeutic index, which means that the difference between an effective dose and a toxic dose is small."
Indeed, it is relatively easy to accidentally overdose on acetaminophen. The compound is found in headache and cold medicine. When people get sick, they often take a combination of over-the-counter drugs to relieve symptoms. Because many of these products contain acetaminophen, it isn't too difficult to overdose, which can cause liver damage. The overdose risk is even higher for elderly people or patients who take prescription painkillers, many of which also contain acetaminophen.
Intentionally or accidentally, about 44,000 to 78,000 Americans end up in the emergency room every year because of Tylenol overdoses.
ψ-Glutathione: Protecting Mice from Acetaminophen
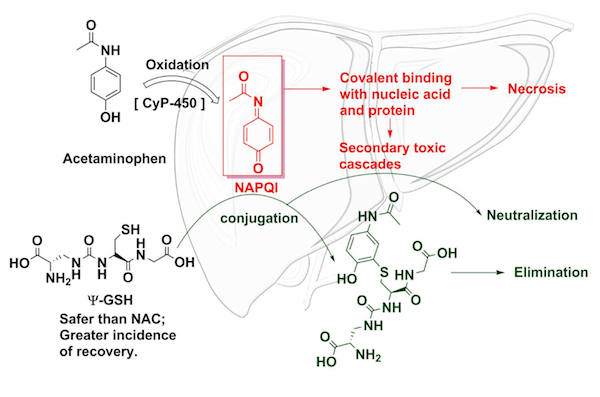 The body metabolizes acetaminophen into various compounds, most of which are harmless. However, one of the products, called NAPQI, is toxic to the body. Under normal conditions, a tripeptide (a molecule made of three amino acids) called glutathione mops up the mess. But when somebody consumes too much acetaminophen, the body is overwhelmed. NAPQI then damages DNA and proteins and leads to liver cell death.
The body metabolizes acetaminophen into various compounds, most of which are harmless. However, one of the products, called NAPQI, is toxic to the body. Under normal conditions, a tripeptide (a molecule made of three amino acids) called glutathione mops up the mess. But when somebody consumes too much acetaminophen, the body is overwhelmed. NAPQI then damages DNA and proteins and leads to liver cell death.
Currently, the standard treatment for acetaminophen overdose is a drug called N-acetylcysteine (NAC), which provides the fuel necessary for the body to create more glutathione. (Glutathione itself cannot be administered orally to patients because the body metabolizes it.) NAC is nearly 100% effective at saving lives and preventing liver damage but only if administered within eight hours of overdose. NAC can also cause strange immunological side effects. For these reasons, a team of scientists from the University of Minnesota went about finding a better antidote.
They may have discovered one in Ψ-glutathione. (Ψ is the Greek letter "psi.") This compound is structurally similar to glutathione but different enough that it is not metabolized by the body. Instead, it latches on to the toxic NAPQI, neutralizing the threat. Additionally, it is more effective than NAC at reducing liver toxicity in mice four hours after the acetaminophen overdose.
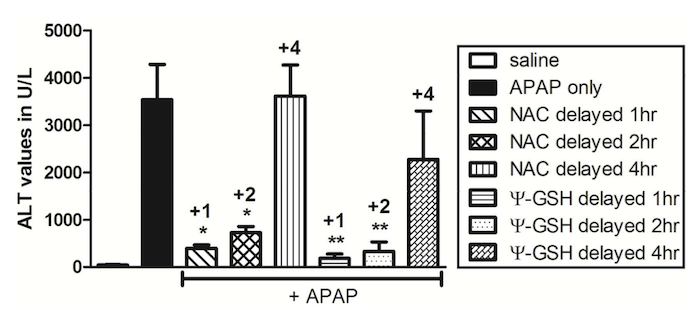
As shown above, mice given an acetaminophen overdose (APAP only) had extremely high liver toxicity (as measured by the level of an enzyme called ALT). Treatment with NAC substantially reduced liver toxicity when given one or two hours after the overdose. However, if NAC was given four hours after the overdose, there was no reduction in liver toxicity, and 80% of the mice died. On the other hand, Ψ-glutathione demonstrated liver protection even up to four hours after the overdose3. Importantly, none of the mice died.
More Work Needed
This was a small but incredibly promising mouse study. The researchers have already demonstrated that Ψ-glutathione is not toxic in mice. The next step may be clinical trials, in which the authors must determine if the new drug works better and produces fewer side effects than NAC in humans.
Notes
(1) Here's the video clip. Here's a transcript.
(2) It seems that the term "acetaminophen" is used more often in the United States, while "paracetamol" is more common elsewhere in the world.
(3) After four hours, the toxic NAPQI likely has already damaged liver cells. So the protective effect of Ψ-glutathione might be due to an ability to block oxidative stress.
Source: Swati S. More, Jaime Nugent, Ashish P. Vartak, Steffan M. Nye, and Robert Vince. "Hepatoprotective Effect of ψ-Glutathione in a Murine Model of Acetaminophen-Induced Liver Toxicity." Chem. Res. Toxicol., Article ASAP. Published: 6-February-2017. DOI: 10.1021/acs.chemrestox.6b00291