 An op-ed in the November 9 New York Times, entitled Making Chemistry Green, by Robert S. Lawrence and Rolf U. Halden could have been entitled Green in Chemistry. based on some rather obvious errors.
An op-ed in the November 9 New York Times, entitled Making Chemistry Green, by Robert S. Lawrence and Rolf U. Halden could have been entitled Green in Chemistry. based on some rather obvious errors.
With a little poking around, we found out why. Here are the academic credentials for the authors:
Lawrence: MD , Harvard Medical School , 1964
Halden: M.S. and Ph.D. in civil engineering (with a concentration in environmental engineering) from the University of Minnesota; Master s in biology from the Technical University of Braunschweig, Germany.
Notably absent is the mention of any degree whatsoever in chemistry something that would seem to be the bare minimum for writing about it. It shows.
ACSH s Dr. Josh Bloom, who does know a thing or two about chemistry points out some errors:
We should regulate chemicals as we understand them: in groups. Instead of regulating one compound at a time..
Bloom: No: that s exactly what we should not do. Within classes of chemicals, very small changes in the molecule can lead to significant differences in properties.
Here s an example from their own editorial grouping triclosan and triclocarban together gives the impression that they are the same. Not true.
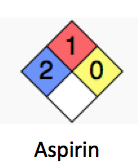
A simple example of this is found by using the safety diamonds from the National Fire Prevention Association. The NFPA safety system for chemicals has been in use since the 1950s, and now contains toxicity and flammability data for many thousands of chemicals. It is qualitatively useful, so that first responders can have an idea of what they are dealing with in the event of fire or accident. Here is their hazard rating of chemicals:
(Ignore the red (flammability) and yellow (reactivity) boxes they are not relevant.)
Health (blue box)
0 Poses no health hazard, no precautions necessary and would offer no hazard beyond that of ordinary combustible materials
1 Exposure would cause irritation with only minor residual injury
2 Intense or continued but not chronic exposure could cause temporary incapacitation or possible residual injury
3 Short exposure could cause serious temporary or moderate residual injury
4 Very short exposure could cause death or major residual injury
Right away, you can tell that triclosan and triclocarban are not the same:
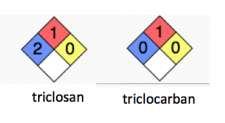
Triclocarban is in category zero completely harmless, while triclosan is in category two safe except in the event of overexposure, and even then, not terrible.
To put this in perspective, here are a couple of chemicals of interest:
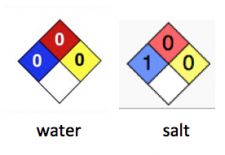
It is immediately obvious that triclocarban is as safe as water, and a little more so than salt. Doesn t sound so awful, does it?
Category 1 speaks for itself, but what about category 2. How bad is that?
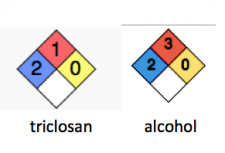
Yep, triclosan is in the same group as alcohol, except alcohol burns much more easily (red box).
Lawrence and Halden: While triclosan and triclocarban are not DDT ¦
Dr. Bloom adds, Don t be so sure:
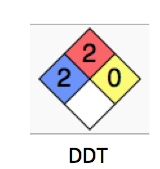
DDT, triclosan, and alcohol are are all category 2 chemicals. I doubt anyone will stop drinking because of this, but perhaps you can sleep a little better at night. And maybe reconsider your concept of DDT as well.
Dr. Bloom continues, Their chemistry is, however, partially correct. Chemicals containing multiple halogen atoms (chlorine, fluorine, bromine) do tend to bioaccumulate because in most cases they are inert to physiological and environmental conditions that metabolize or degrade most other types of chemicals. But this alone does not make them harmful. You can see this easily from the difference of triclosan and triclocarban. There are many other examples.
Finally, the writers say, Synthetic chemicals are vital to our society. But we should be doing everything possible to make sure they are safe.
Dr. Bloom says, this is correct, but misleading. He says, Perhaps the most common, simplistic fallacy regarding chemicals is that there is an invisible wall between synthetic (poison) and natural (harmless) chemicals. This is just plain wrong. Neither your body nor your rose bushes know whether nature or a factory made a chemical that it is exposed to. It doesn t matter. Chemicals are like people they are all different.
Dr. Bloom does agree with one of the authors points though why either or these antibacterial agents are needed in soap at all. He says, If you think about the common diseases that people catch every year, cold, flu, stomach flu, these are all viruses, so antibiotic soap will do nothing more than any other soap. And I also wonder what bacteria that these biocides will protect us from that a normal hand washing will not. Safe or not, if there is no benefit to any ingredient, why use it?
ACSH Dr. Gil Ross had one further comment addressing these authors qualifications: When they compare these 2 antibacterials toxicity to DDT, they truly expose their complete lack of understanding of basic toxicology and its application to human health. DDT, despite the common mythology first promulgated by Rachel Carson s Silent Spring , is entirely harmless for humans at just about any conceivable exposure. Its ban in 1972 by our EPA was and remains purely ideological and has been hugely harmful in terms of needless suffering and death from malaria.
We apologize if this has given you a headache. If so, you might want to try some of this: