
(This companion piece discusses the chemistry of erythritol and other sugar articles. In his article, Dr. Chuck "Chuckles" Dinerstein discusses the potential risks of the artificial sweetener erythritol based on a single study.)
It never ends. There are 12 approved sugar substitutes in the US; six are icky artificial chemicals that, according to some schmoe or other, are supposed to cause cancer. (The King of Bogeymen is aspartame, a harmless artificial sweetener that people recoil from while downing three gin and tonics every night. I recently wrote about how alcohol is a far greater cancer risk than aspartame. See Worrying About the Wrong Carcinogen: Alcohol vs. Aspartame.)
Now, caught in the revolving door of scary artificial sweeteners, is erythritol, a naturally occurring sugar alcohol. (My beloved colleague, Dr. Chuck "Chuckles" Dinerstein, has written a companion piece on some recent information linking erythritol to blood clots. You can read it here.)
Let's clear up the terms
'Sugar alcohol' is an inherently confusing term. Are they sugars? Sort of because they're sweet (1). Are they alcohols? Yes, because they contain multiple hydroxyl (alcohol) groups. Both are partially correct, but it takes a bit of chemistry to explain the term properly. And who could possibly be better for this monumental task than our always-ornery friends Steve and Irving, long-time denizens of hell and (reluctantly) the hosts of The Dreaded Chemistry Lesson From Hell®? They finally got some long-overdue recognition and were featured at the halftime show of the prestigious 2023 Wasabi Fenway Bowl (2) !

Steve (left) and Irving were honored at the 2023 esteemed Wasabi Fenway Bowl
Image: Wikimedia Commons
Hang on. Do that many people really attend the Wasabi Bowl? I'm suspicious. Why am I thinking the following might be more accurate?

Image: Stockbridge
What is a sugar?
Sugars (hundreds occur naturally) are one type of carbohydrate (consisting of carbon, hydrogen, and oxygen atoms). In particular, all sugars contain multiple hydroxyl (aka alcohol) groups and are sweet-tasting and water-soluble.
There are about 20 naturally occurring sugars that have a common feature. The general formula below shows that they exist in two forms – open and closed. The open forms contain an aldehyde group (red asterisk). Why am I torturing you with this minutia? It's an explanation of why sugars and sugar alcohols are different. Bear with me. Glucose, the most important, is used as an example (Figure 1).
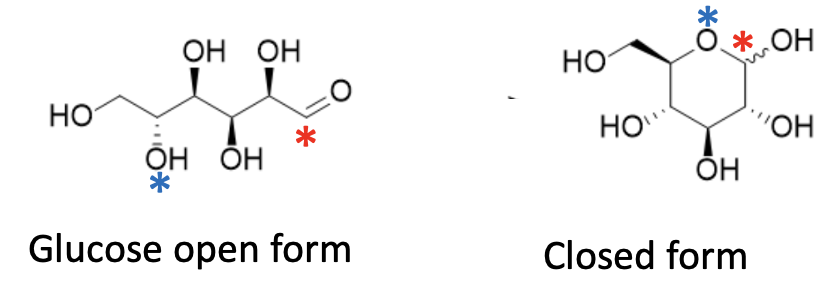
Figure 1. Glucose interconverts at lightning speed. The open form contains an aldehyde (left, red asterisk) and an alcohol group (left, blue asterisk), which react reversibly with each other, while the closed form has neither. The asterisks in the closed form correspond to the same atoms in the open form.
Sugars vs. sugar alcohols - both sweet. One might keep you near a bathroom
Sugars (about 4 calories/gram) are absorbed quickly in the small intestine and are used by the body. Sugar alcohols, on the other hand, are usually absorbed more slowly and often incompletely, so they have little impact on blood sugar and have fewer calories. Some, like erythritol, pass through the body largely unchanged, while others are fermented by bacteria in the large intestine, which can lead to digestive discomfort. (This is putting it mildly.) Sorbitol, which is close in chemical structure (Figure 2) to glucose, is notorious for this.
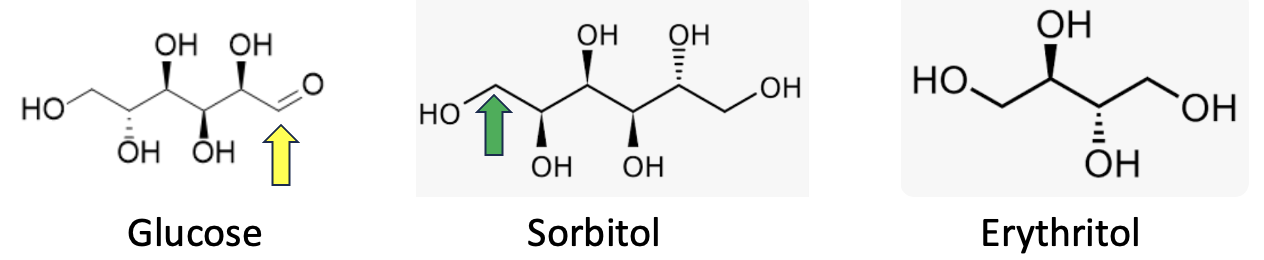
Figure 2. Note that in sugar alcohols the aldehyde group (yellow arrow) has been reduced (two hydrogen atoms added) to form an alcohol group (green arrow), which is why this class of compounds is also referred to as reduced sugars. In organic chemistry, reduction means the addition of hydrogen. Sorbitol has two more hydrogen atoms than glucose.
Bottom line
The body does a poor job of handling sugar alcohols, which are structurally different albeit subtly from sugars. This is why they have few or no calories. But there are tradeoffs. Some people become quite ill when they consume these sweeteners. Aside from that they are generally regarded as safe (except perhaps for your underwear). At least until now. The latest scare: Does erythritol cause clotting and contribute to stroke risk?
Time to get out
Let's turn this over to Dr. Dinerstein. He's both sweet and easy on the intestines.
Here's Chuckie!

Original image: Wikipedia
NOTES:
(1) Most but not all organic compounds that have multiple hydroxyl groups are sweet. One dangerous example is ethylene glycol, which is used as an antifreeze in cars. This is the chemical that kills pets; they lick it because of its sweet taste.
(2) On the off chance that you missed it, the Boston College Eagles won the 2023 Wasabi Fenway Bowl, beating the SMU Mustangs 23-14. Beyonce did not perform during the halftime show.



