
"Forever chemicals"– good, bad, or otherwise?
Per- and polyfluoroalkyl substances (PFAS) and perfluorooctane sulfonic acid (PFOS), aka, "forever chemicals," have been widely reported in the news recently. These chemicals are suspected of being carcinogens. (In organic chemistry, "per" means that all hydrogen atoms in the molecule have been replaced by another atom. "Poly" means that most of them have.)
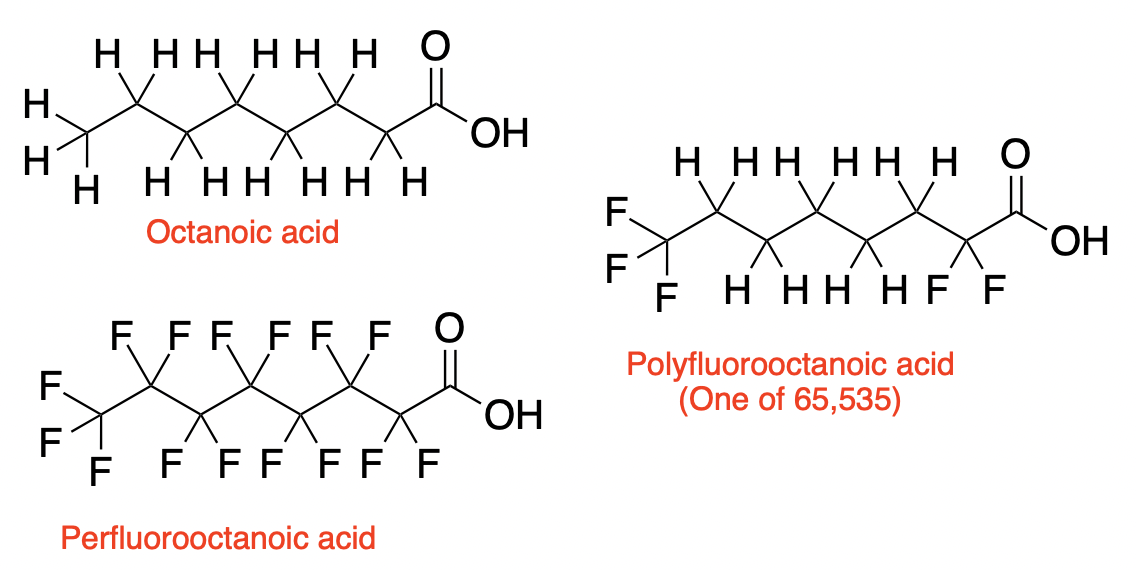
(Left) When all the alkyl hydrogen atoms of octanoic acid (top) are replaced by fluorine atoms (bottom), the compound perfluorooctanoic acid (PFOA) becomes "indestructible." (Right) There are 65,535 possible compounds that make up the "polyfluorooctanoic acid family" Blame ChatGPT for this.
It's not precisely history repeating itself, but about 75 years ago, another class of forever chemicals, PCBs (polychlorinated biphenyls), made its way into the environment when General Electric began dumping huge quantities of these chemicals into the Hudson River over a 30-year period. Despite an enormous dredging project that began in 2009, it's been impossible to get them out of the river, despite an enormous dredging project that began in 2009. Because they are chemically inert PCBs had several industrial uses, including electrical transformers, hydraulic fluid, lubricants, and coatings. That's all well and good, but dumping the stuff into the Hudson was anything but. It will be impossible to remove all of the PCB waste. That waste will be with us forever.
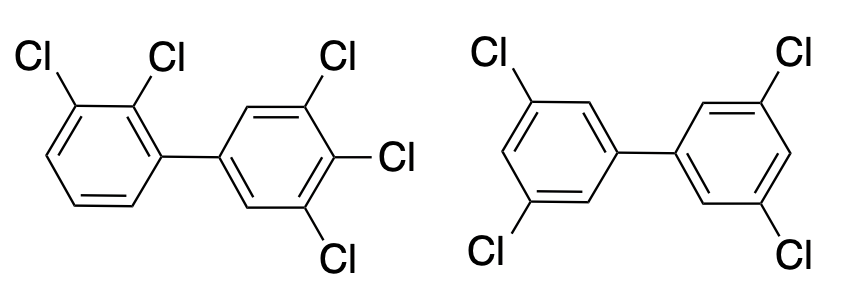
Two examples of polychlorinated biphenyls (PCBs). There are 209 possible PCBs, of which about 130 have been synthesized.
What makes certain chemicals last "forever?"
"Forever chemicals" are the exception, not the rule. Of the estimated 20 million known organic chemicals (1), roughly 5,000–almost all synthetic (2) — are "forever chemicals." These chemicals share certain properties that make them persist: chemical stability and resistance to enzymatic degradation (metabolism). This is why they were created in the first place.
Chemical stability
Organic chemicals that contain only hydrogen and carbon (hydrocarbons) are prone to chemical degradation and combustion. This is also true for chemicals that include carbon, hydrogen, and heteroatoms (3), such as nitrogen, oxygen, and sulfur. Halogens are also heteroatoms, but they have a different impact on the properties of the chemical; the molecules become more resistant. Here's an example. Here's an example.
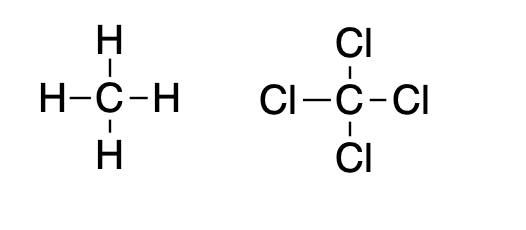
Methane (left), the main component of natural gas is (duh) quite flammable but when its four hydrogen atoms are replaced by chlorine the result is carbon tetrachloride, which is so non-flammable that it was used in fire extinguishers in the mid-20th century. Carbon tet is rather toxic, so while it may put out fires there are better choices.
Likewise, while benzene is both combustible and also chemically reactive–it reacts with hundreds of chemical reagents (4), hexachlorobenzene is chemically inert and highly resistant to enzymatic degradation.
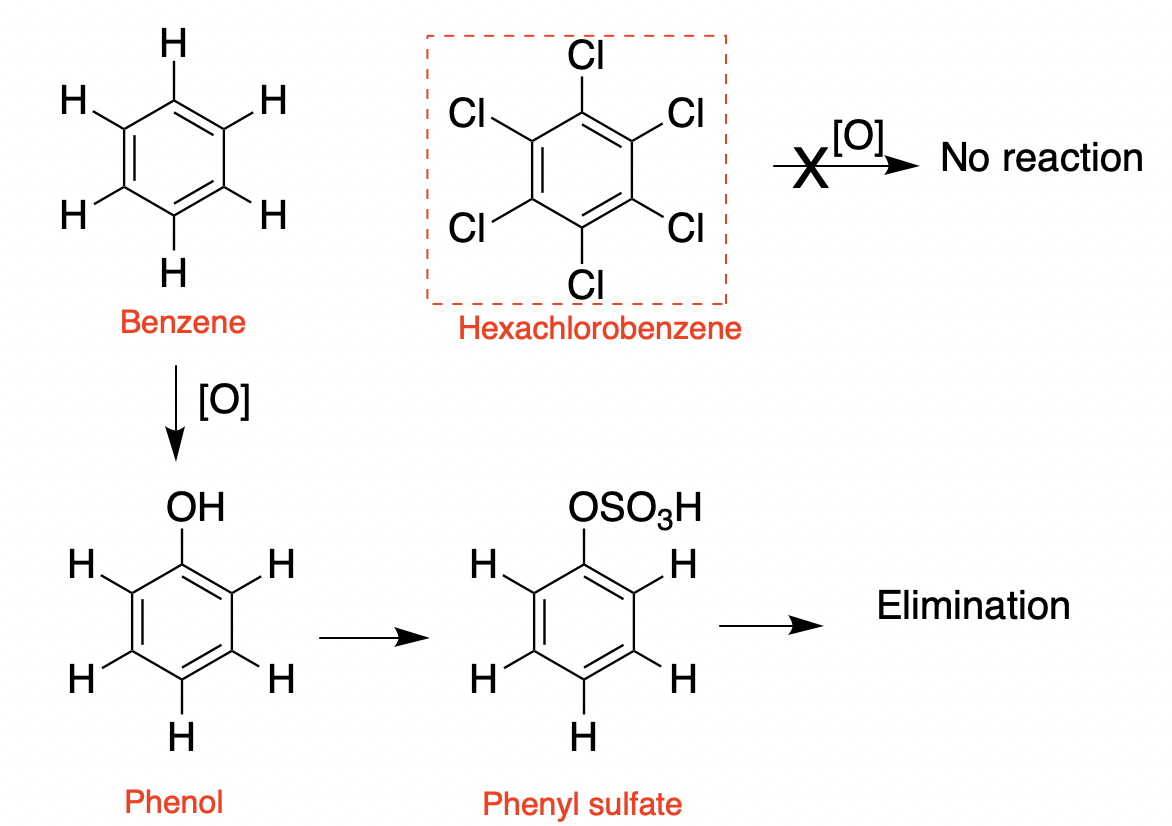
(Left) Benzene can be oxidized by CYP enzymes to form phenol. The hydroxyl group then provides a handle for further metabolism, converting it to the water-soluble phenyl sulfate, which is readily eliminated. (Right) Hexachlorobenzene cannot undergo this oxidation and remains unchanged, This is why it accumulates.
Whatever chlorine can do, fluorine can do better.
The addition of fluorine atoms makes chemicals even more stable; it is no coincidence that PFOA and PFAS were synthesized for specific purposes like non-stick cookware, firefighting foam, and water-repellant fabrics. This is because the carbon-fluorine bond is the strongest of all carbon-heteroatom bonds. Here's the reason:
- You don't want to know
- OK, if you do, the carbon-fluorine is the shortest of carbon-heteroatom bonds. Short bonds are strong bonds. Why?
- You don't want to know
Enzymatic and metabolic stability
Chemical stability is the ability to resist reactive chemicals like chlorine and sulfuric acid. Enzymatic stability is the ability to withstand biological metabolism, typically facilitated by enzymes such as CYPs and proteolytic enzymes like pepsin and chymotrypsin. There is a significant overlap between the two processes; chemically stable compounds also tend to be biologically stable as well.
This is why PFAS and PCBs persist in the environment and also bioaccumulate in living species. Enzymatic reactions are simply organic chemistry reactions that are greatly facilitated by the structure and properties of enzymes. The desired properties of these chemicals go hand in hand with their persistence in the environment and your body.
Bottom line
When companies began producing inert chemicals like PFAS and PCBs, it was almost inevitable that these substances would persist in the environment. Does this mean that they should never have been made? This question is not as straightforward as it seems. While we could do without grease-resistant pizza boxes and water-resistant makeup, PFAS are essential for making critical medical devices such as catheters, implants, surgical sutures, and dialysis equipment.
One way to view this is that the use of these chemicals determines whether synthesizing them was a "good" idea. Regardless of whether these chemicals are proven to be harmful to human health (this remains unknown), perhaps we could have been a little more selective in their use. No, a pizza in a PFAS-treated box isn't going to harm you, but neither would a greasy box. Were these chemicals overused? Maybe.
NOTES:
(1) The definition of an organic chemical isn't clear-cut. It usually refers to any chemical containing at least one carbon atom and one carbon-hydrogen bond. Some chemicals are not easily characterized, such as CCl4, which is considered to be "organic." Maybe. Both carbon monoxide and carbon dioxide are inorganic, even though they contain carbon. The definition of these few examples is largely irrelevant.
(2) With very few exceptions, virtually all fluorine-containing organic chemicals are synthetic. A handful are made by marine algae. Organofluoro compounds found in nature are few and far between.
(3) Heteroatoms are atoms in an organic molecule that are not carbon or hydrogen. These include oxygen, sulfur, nitrogen, phosphorous, halogens, others.
(4) A reagent is a chemical that is used to promote a chemical reaction. For example, chlorine is the reagent used to convert benzene into chlorobenzene.
(5) This does not mean that these highly stable chemicals are necessarily harmful. As I have written hundreds of times, the presence of a chemical tells us little about its harm. Harm depends on innate chemical toxicity as well as exposure (dose).



