Here's the mission statement from the website of Physicians for Responsible Opioid Prescribing (PROP):
PROP’s mission is to reduce opioid-related morbidity and mortality by promoting cautious and responsible prescribing practices.
But is PROP really interested in preventing drug-related deaths or merely focused on driving opioid drugs and the companies that make them into the ground? A new JAMA study suggests that the latter may be more accurate. This is because almost all opioid medications combine the actual painkiller and acetaminophen (Tylenol). And, as you'll see, merely focusing on cutting the opioid has a subtle downside since it ignores the potential damage from the acetaminophen – a known liver toxin, something I wrote about in 2019.
Reducing the amount of acetaminophen in these combination pills profoundly affects the safety of drugs like Vicodin and Percocet, something that PROP either does not know or cares about. Interestingly, the pills in question are considerably safer now than only 12 years ago. Why? In 2011 the FDA limited the amount of acetaminophen (Tylenol) that could be included in a Vicodin or Percocet pill to 325 mg per pill (1). Before this change, a single opioid combination pill could contain as much as 750 mg of acetaminophen.
The JAMA paper examined how much safer opioid pills are since 2011 and also provides strong evidence that the reduction of the dosage of acetaminophen, supposedly the benign ingredient in combination pain medications, was responsible. The difference is sobering, something that Babak J. Orandi, MD, Ph.D. and colleagues point out in a new article titled "Association of FDA Mandate Limiting Acetaminophen (Paracetamol) in Prescription Combination Opioid Products and Subsequent Hospitalizations and Acute Liver Failure."
The authors asked the question:
Was the US Food and Drug Administration (FDA) mandate to limit acetaminophen (paracetamol) to 325 mg/tablet in prescription combination acetaminophen and opioid medications associated with a decrease in hospitalizations for acetaminophen and opioid toxicity and a decline in the proportion of acute liver failure (ALF) hospitalizations due to acetaminophen and opioid toxicity?
Orandi, et.al., JAMA. 2023;329(9):735-744. doi:10.1001/jama.2023.1080
The answer is a clear yes. Here's why.
The group's analysis consisted of a review of hospitalization data from acetaminophen and opioid toxicity between 2007 to 2019 and also acute liver failure between 1998 to 2019. The hospitalization data were obtained from the National Inpatient Sample (NIS), a large hospital database hosted by the Agency for Healthcare Research and Quality (AHRQ). The liver toxicity data were obtained from the US Acute Liver Failure Study Group (NIH).
The results
1. Hospitalizations
Of the more than 474 million hospitalizations in the NIS database (2007-19), almost 40,000 involved opioid and acetaminophen toxicity. The Orandi group compared the number of hospitalizations before and after the 2011 FDA ruling was announced (Figure 1).
During the 2007-2010 time period (prior to the FDA's action), hospitalizations increased by 11%. After the FDA's announcement (2011-19), the opposite was observed: an 11% decrease in hospitalizations. Keep in mind that it was the acetaminophen that was reduced, not the opioid, and this alone made the combination drugs safer.
These numbers are more significant than they seem
In many studies, an 11% difference might be dismissed as a statistical blip but with a database of 474 million hospitalizations; the difference is real and significant (p < 0.001). Also, keep in mind that a "typical" opioid pill before 2011 might contain 5-10 mg of the narcotic and 500 mg of acetaminophen (this is what Vicodin 10/500 or Percocet 5/500 mean), so huge doses of acetaminophen were not being used before 2011, just a bit larger.
It is also important to note that the significant difference in hospitalizations arose from a relatively small reduction in the acetaminophen dose – 500 mg (2) to 325 mg per pill. I find it astounding that a per-pill decrease in dose of 175 mg of a supposedly safe and benign drug – one gram (3) per day assuming a daily dose of 6 pills – would have any impact on hospitalizations, especially since it is reasonable to assume that hospitalized patients did not take additional acetaminophen on their own.
2. Liver Failure
The data from the US Acute Liver Failure Study Group showed a similar trend. Before the FDA announcement, acute liver failure (ALF) cases increased by 7% per year; afterward, it decreased by 16% annually (Figure 1).
"A total of 2,631 ALF cases were identified in the ALFSG from the first quarter of 1998 through the third quarter of 2019; 465 of these involved acetaminophen and opioid toxicity"
Orandi, et.al., JAMA. 2023;329(9):735-744. doi:10.1001/jama.2023.1080
The authors' conclusion:
The FDA mandate limiting acetaminophen dosage to 325 mg/tablet in prescription acetaminophen and opioid products was associated with a statistically significant decrease in the yearly rate of hospitalizations and proportion per year of ALF cases involving acetaminophen and opioid toxicity.
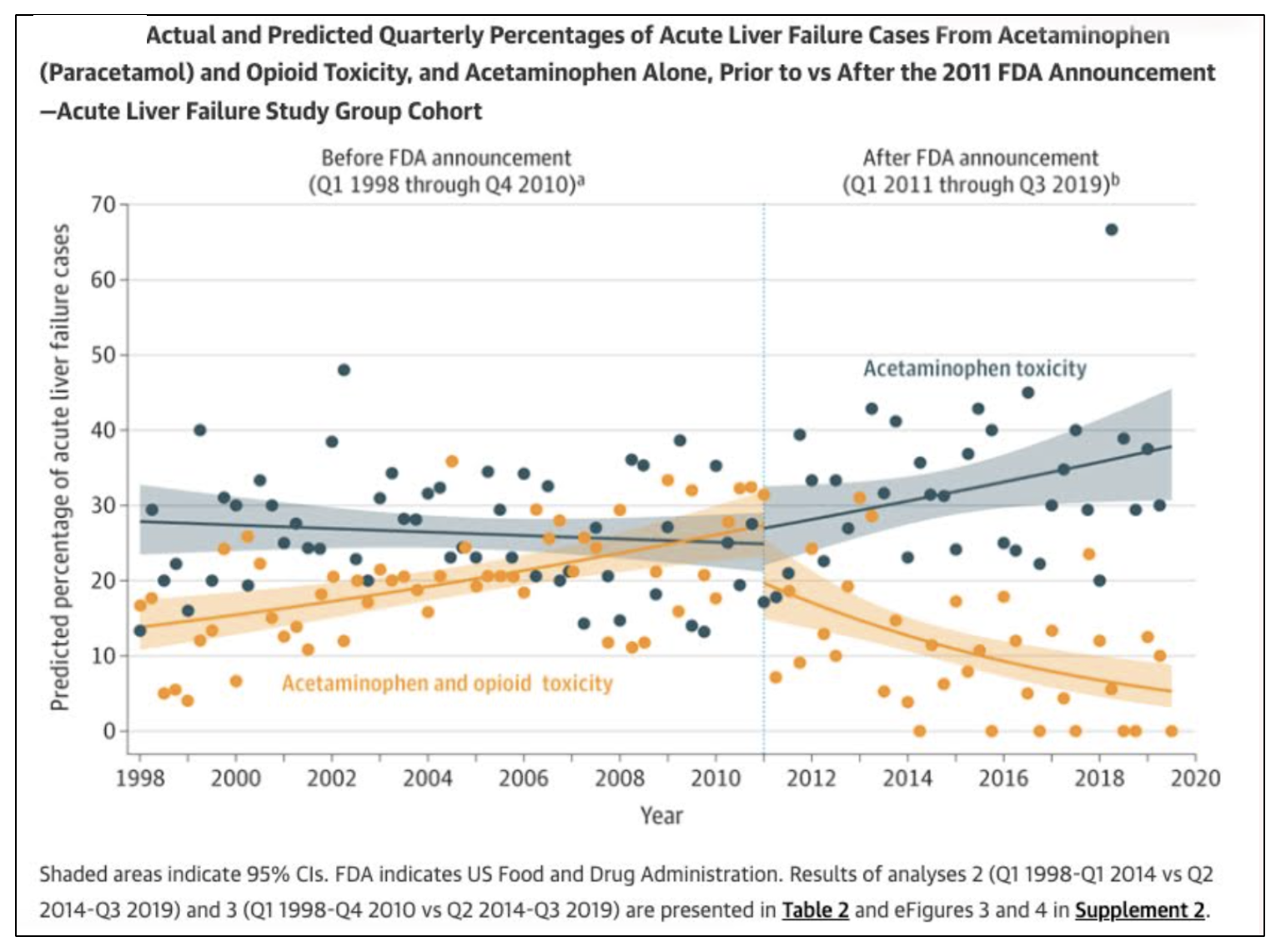
Figure 1. Acute liver failure cases before and after the FDA's 2011 decision to reduce the dose of acetaminophen in combination pills. The solid gray and orange regions of the graph indicate a 95% CI.
Note that between 1998 and 2011, acute liver failure (ALF) from acetaminophen alone (gray) was largely unchanged. However, ALF cases from acetaminophen plus an opioid increased by 11% (orange). By contrast, after 2011, ALF cases from acetaminophen plus an opioid dropped significantly, showing that even a relatively small decrease in acetaminophen resulted in a 17% decrease in ALF. Perhaps most interesting is the increase in ALF from acetaminophen alone after 2011. This can reasonably be interpreted as people being forced to turn to acetaminophen (only) because obtaining drugs like Vicodin became more difficult.
A Final Note
I have seen no evidence that PROP cares one bit about anything except addiction, and only that caused by legally prescribed opioid drugs, not alcohol, cocaine, or methamphetamine. Have they ever expressed any concern about people suffering from pain? How about the toxicity of acetaminophen – the drug used in truckloads as a "substitute" for real pain medicine? Do they care about this?
PROP’s mission is to reduce opioid-related morbidity and mortality by promoting cautious and responsible prescribing practices.
If this were true, then the group should also be concerned about the acetaminophen content in the pills, not just reducing the dose of the opioid component.
Try a Google search using the terms "physicians for responsible opioid prescribing" and "acetaminophen toxicity" and look for any instances where the group expresses any concern.
There isn't a single instance.
NOTES:
(1) Manufacturers had until 2014 to comply with the new FDA directive.
(2) Prior to 2011, the amount of acetaminophen in opioid painkillers typically ranged from 325 mg to 750 mg. 500 mg was a common dose.
(3) This amount (one gram) cannot be a coincidence. In 2011 the FDA also recommended that the maximum daily dose be lowered from 4,000 milligrams to 3,000 milligrams for adults who take the medication regularly.