The COVID-19 pandemic has been one of the most devastating events in public health in the U.S. over the last half-century. It’s also the most scrutinized health emergency in history. But one aspect of the outbreak that has gone largely unnoticed is the transmission of the SARS-CoV-2 virus back-and-forth between humans and other mammals and the threat that it poses. Even as the virus has faded from the headlines, animal-human transmission is on the rise. Failing to fully understand what is happening could lead to another major health crisis.
The two-way transmission occurs via processes known as spillover and spillback. Spillover is the transmission of a pathogen from an animal to a human, which in most cases does not give rise to human-to-human transmission. Spillback (reverse spillover) is the transmission of a pathogen from humans to animals, which may or may not give rise to illness in the animal. For example, the SARS-CoV-2 virus is known to have been transmitted from humans to mink, dogs, cats, bears, various zoo animals, and now deer.

Credit: NorthJersey.com
At least 30 million white-tailed deer are found across the U.S. Since 2021, evidence of SARS-CoV-2 in white-tailed deer populations has been mounting. From Iowa to Pennsylvania, the virus has become rampant. How do we know this? By happenstance — from samples taken from about 1% of the hundreds of thousands of deer harvested by hunters or killed in car collisions each year. Deer are tested for chronic wasting disease (CWD), a prion disorder that various states keep under surveillance, so as the human version of the pandemic raged on, this program enabled researchers to access samples from deer across the continent.
Previously dominant SARS-CoV-2 “variants of concern” like alpha, gamma, and delta, which are no longer commonly found in human populations, continue to cycle throughout our wild fauna, raising the possibility of virus spillover to humans.
But there is even more to this story that is cause for concern.
A closer look at the explosion of COVID in deer from 2021 to 2022 shows that not only were formerly prevalent variants of concern alive and well and living in our backyards unbeknownst to us, but they were mutating in ways that no one could have predicted. These deer SARS-CoV-2 genomes have diverged significantly from human-derived sequences, with some strains having accumulated more than 70 nucleotide mutations across their genome. The delta variant has changed the least, presumably because it emerged later in the pandemic.
A similar study published last year of white-tailed deer in Ontario, Canada, identified a highly divergent lineage of SARS-CoV-2 (B.1.641) that is highly divergent from other known SARS-CoV-2 lineages, with 76 mutations (including 37 previously associated with non-human mammalian hosts). Their analysis suggested that evolution and transmission occur in deer and that there is a shared ancestry with the mink-derived virus. It also revealed “an epidemiologically linked human infection.” In sum, their findings “provide evidence for sustained evolution of SARS-CoV-2 in white-tailed deer and of deer-to-human transmission.”
It is unsurprising that white-tailed deer are a reservoir for human viral pathogens. They are ubiquitous and have long occupied a comfortable niche at the intersection between our wild places and areas of human habitation. In many parts of North America, deer also inhabit our backyards. They eat from our birdfeeders, munch on our shrubs, and graze on our lawns. We have inadvertently developed outdoor landscapes that are perfect for them. It is not surprising that, like many other mammals with ACE2 receptors, the cellular binding site at which the SARS-CoV-2 virus enters cells, deer can carry COVID.
It is disturbing that SARS-CoV-2 has been circulating in deer in parallel with the human pandemic. The high mutation rate suggests that a new variant could eventually emerge and return to the human population.
Our experiences with infectious diseases of local wildlife have sometimes been problematic. We have not managed to eradicate Lyme disease, West Nile virus, plague, rabies, and coronaviruses MERS and SARS-CoV, among others. We stumbled onto the recent accounts of COVID in deer because we had a surveillance program for a different disease (CWD) entirely.
The potential for spread of SARS-CoV-2 infections through many mammal species in the wild raises the specter of deer becoming a SARS-CoV-2 reservoir — a permanent home for the virus and a regular source of outbreaks in other animals, including humans. Camels, for example, are a natural reservoir of the coronavirus (MERS-CoV) that causes Middle East Respiratory Syndrome, which occasionally jumps to people.
Once established in deer, SARS-CoV-2 could mutate, evolve and possibly recombine with other coronaviruses, giving rise to new strains of the virus with novel properties such as greater transmissibility or virulence.
Credit: Semantic Scholar
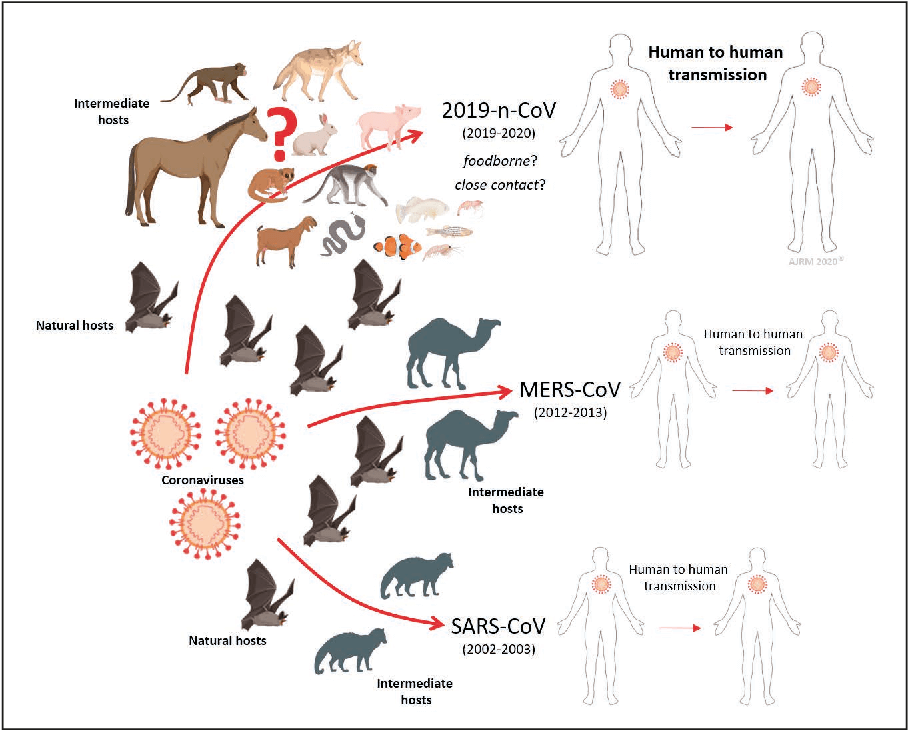
Such concerns are neither new nor limited to coronaviruses. A 2015 article in Nature Medicine by an international group of researchers described the disease potential of a SARS-like virus, SHC014-CoV, which was then circulating in Chinese horseshoe bat populations. They concluded that there was “a potential risk of SARS-CoV re-emergence from viruses currently circulating in bat populations.” Similarly, poultry provides a reservoir for H5N1 bird flu, which has swept poultry farms worldwide in recent years. There have been reports of several infections transmitted to humans, but no human-to-human transmission has been detected.
Where does all this leave us? We need to continue to perform coronavirus surveillance of animals in the wild and be prepared for the emergence of new pandemic viruses. Also important is the development of additional anti-viral and immunity-boosting drugs and the maintenance of infrastructure for the rapid development and scale-up of new vaccines.
Note: An earlier version of this article was published by the Genetic Literacy Project.
Dr. Kathleen L. Hefferon is an instructor in microbiology at Cornell University. Find Kathleen on Twitter @KHefferon




