As Professor Katherine Seley-Radtke (1) and I wrote last July in an op-ed in the Baltimore Sun, "Vaccines might not be the only way to treat COVID-19." At that time – four months before the results of the Pfizer-Biotech and Moderna vaccines were announced – no one knew whether an effective vaccine against COVID was even a realistic possibility. Certainly, no one could have imagined that the mRNA vaccines would be 95% protective against COVID, or they would lose effectiveness with time, or that a "super variant" would take over the world. Or that we would be getting third shots six months after the second shot.
After the good news about the vaccines, research on antiviral drugs dropped off the front page. After all, it was "simply" a matter of getting the world vaccinated, and COVID would be a distant memory. Of course, the virus had other ideas called variants. Now, the drug candidates are back on the front page as it has become obvious that a second weapon against the pandemic has become important, maybe even critical (2).
As a former medicinal chemist (3) who worked for more than a decade in antiviral drug discovery, I was quite interested in clinical trials of direct-acting antiviral drugs, especially those that could be administered orally (pills, for the most part). Why? In the 1990s, I was involved in the research in both HIV and hepatitis C (HCV) – the two most important viral infections on the planet at that time. These tough infections would eventually be controlled (HIV) or cured (HCV) by drugs that inhibited specific steps in the viral replication cycle (4). Indeed, in my opinion (and I'm not alone), the decades-long campaigns against both infections represent some of the most important and successful work in the history of drug discovery.
Will medicinal chemists be able to recreate these successes and discover effective drugs against COVID? It's too soon to say, but I'm optimistic. The strategy used to inhibit HIV was largely paralleled in the effort to cure HCV. Now our knowledge base of how to stop viruses using medicines is being used to design anti-COVID drugs. It might not work, but it should. Here's why.
Earlier research says yes
From the time HIV was first discovered (1981), it took 14 years for Roche's saquinavir (brand name Invirase), the first member of a class called HIV protease inhibitors and the first effective AIDS drug, to hit the pharmacies. For the first time, AIDS deaths in the US began to decline.
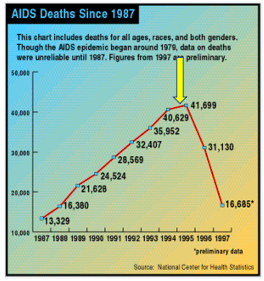
AIDS deaths in the US began to decline in 1995 following the approval of saquinavir (yellow arrow). Source: National Center for Health Statistics, Wikipedia
Perhaps more important is that saquinavir represented the first proof of principle – that inhibiting an essential step in HIV replication will inhibit replication of that virus. In all, ten HIV protease inhibitors were approved; none are used today. They have all been replaced by better drugs that operate by different mechanisms and have fewer side effects. But these efforts were not wasted.
Protease inhibitors for hepatitis C - A groundbreaking but short-lived success
Following the discovery of hepatitis C in 1989, dozens of companies tested millions (5) of random chemical compounds looking for an HCV drug. They all bombed. However, both Vertex and Merck-Schering, following the same strategy used for HIV, designed HCV protease inhibitors, which resulted in the approval of telaprevir and boceprevir, respectively, in 2011. Although these drugs were the first direct-acting HCV inhibitors and substantially improved the cure rate over the previous standard of care (6), neither Vertex nor Merck reaped the rewards of their efforts. Two years later, Gilead's sofosbuvir (brand name Sovaldi), an inhibitor of viral RNA synthesis, was approved and blew both of them out of the water. Sofosbuvir is now used in combination with other HCV inhibitors as cocktails, with cure rates approaching 100%.
Pfizer's PF-07321332 looks a whole lot like boceprevir
With apologies in advance to non-chemists...

Even non-chemists should be able to see that the chemical structures of boceprevir and PF-07321332 are quite similar. The blue shaded ovals show where the two molecules are identical, and the green oval shows where they are similar. Why does this matter? There are some similarities between the two viruses. Furthermore, some HCV inhibitors were shown to also inhibit the original SARS virus. Although these drugs weren't needed (SARS died out), the rationale of using known HCV inhibitors or variations of them is sound.
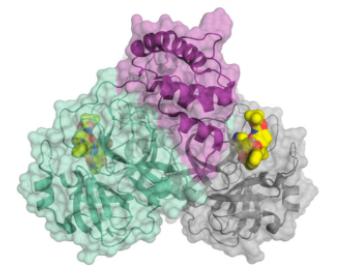
SARS-CoV-2 also requires a protease enzyme to replicate (it is called the main protease or the 3CL protease). Its function is the same as other protease enzymes: to cut up the newly produced long strands of protein made by ribosomes into functional fragments that form the structural and functional proteins needed to make new virus particles. The main protein has been one of the most interesting targets in designing potential SARS-CoV-2 inhibitors. (In March 2020, C&E News published an excellent feature article on the main protein and efforts to inhibit it.)
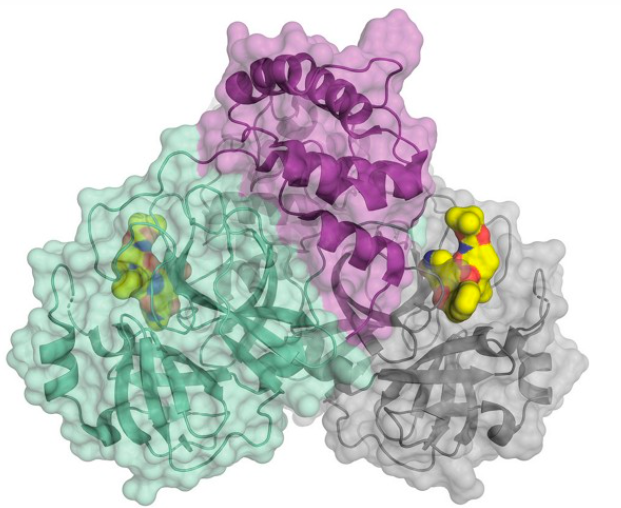
The structure of the SARS-CoV-2 main protease bound to an inhibitor (yellow). Credit: C&E News, H. Tabermann/HZB
Properties of PF-07321332 - Will it make it?
Does PF-07321332 have what it takes to become an orally active drug with sufficient potency to inhibit SARS-CoV-2 in a clinically meaningful way? So far, so good:
- Precedent: Excellent - Operates by a mechanism known to inhibit other viruses
- Potency: Very good - Powerful inhibition of virus replication in cells (EC50 = 77 nanomolar)
- Selectivity: No cellular cytotoxicity at 3 micromolar (Selectivity index > 39)
- Orally active? Yes, especially when given with ritonavir (7)
- Toxicity: Well tolerated in humans (limited data)
Bottom line
I have written many times that no matter how good a drug looks in clinical trials, you'd have to be out of your mind to bet on any of them. Unexpected problems pop up all the time. That said, based on everything I've seen, PF-07321332 has history and precedent behind it and appears to be potent and selective as well as being orally absorbed. This is about as good as a drug can look based on early-stage trials, and Pfizer is now beginning larger efficacy trials. Yes, things look good (8). But don't bet the house on it.
----------------------------------------------------------------------------------------------------------------------------------------------------
UPDATE 10/1/21
Merck just announced that molnupiravir, a nucleotide drug that inhibits the replication of viral RNA – a common strategy for antiviral drugs – works pretty well. (I've written about it before.) Molnupiravir, if approved would be the first orally active direct-acting antiviral drug for COVID – a significant advance in fighting the pandemic. In a trial of 775 patients with mild-to-moderate COVID-19 who were considered higher risk for severe disease molnupiravir reduced hospitalization by 50%. Merck, which is co-developing the drug with Ridgeback says it plans to seek EUA for molnupiravir.
“It exceeded what I thought the drug might be able to do in this clinical trial...When you see a 50% reduction in hospitalization or death that’s a substantial clinical impact.”
Dr. Dean Li, vice president of Merck Research Laboratories
----------------------------------------------------------------------------------------------------------------------------------------------------
NOTES
(1) Dr. Seley-Radtke Katherine is a professor of chemistry and biochemistry at the University of Maryland, Baltimore County. Her specialties are organic chemistry, medicinal chemistry, and antiviral drug design.
(2) This is not a knock on the vaccines. They performed very well, but the virus didn't cooperate. See "Don't Shoot The Messenger RNA: Blame The Virus, Not The Vaccine."
(3) Medicinal chemists have formal training in organic chemistry, usually organic synthesis. These skills are applied to the drug discovery process where medicinal chemists work to maximize desirable effects of a drug, e.g., potency, and minimize undesirable effects, e.g., toxicity.
(4) It is important to note that both HIV and HCV require cocktails of drugs to eradicate the virus. Single antiviral drugs can be useful (herpes, flu), but viruses can often defeat a single drug by mutation. When two different classes of drugs are thrown at the virus, mutation becomes much more difficult. Whether this will apply to COVID is unknown.
(5) Drug companies routinely run high throughput screens (HTS) as a tool for drug discovery. Robots test collections of chemical compounds (called libraries). The number of compounds tested can be hundreds of thousands, even millions.
(6) The previous standard of care for hepatitis C was interferon-ribavirin. It was hideous (side effects, length of treatment) and not especially effective (~40% cure rate depending on HCV strain).
(7) Ritonavir was originally developed as an HIV protease inhibitor. However, it was found to be a potent inhibitor of the enzyme CYP3A4, which metabolizes the other protease inhibitors. When given with a protease inhibitor, ritonavir increases the bioavailability of the drug.
(8) Two other direct-acting antivirals are in different stages of development. Both are interesting. I have written about molnupiravir. Roche's AT-527, although a bit behind molnupiravir, also looks promising. Both of these drugs are nucleotide inhibitors of RNA polymerase.