
As Professor Katherine Radtke-Seley and I wrote last year in the Baltimore Sun, vaccines alone will probably not be sufficient in the battle against COVID-19. Carl Zimmer, writing for the New York Times, agrees, pointing out that there remains a need for antiviral drugs and that the Biden administration has invested $3 billion to look for a COVID pill. The Antiviral Program for Pandemics will conduct research for novel (not repurposed) drugs against COVID and other viral infections, which are almost certain to emerge.
AIDS Drugs Did Not Simply Fall Out of the Sky
That's all well and good; a low molecular weight (1) antiviral drug would be useful in limiting the severity of disease in people who are not vaccinated or contract a variant not covered by a particular vaccine. But Zimmer's article reflects a perpetual "willful ignorance" by the Times regarding understanding and explaining how and where drugs are discovered and developed. Drugs do not fall out of the sky or, for the most part, out of government laboratories. Zimmer implies that the game-changing drugs against HIV and hepatitis C were simply a result of government-funded research. This is false. Following are some examples.
"A number of other viruses, including influenza, H.I.V. and hepatitis C, can be treated with a simple pill. But despite more than a year of research, no such pill exists to treat someone with a coronavirus infection before it wreaks havoc."
More than a year?? It would be almost impossible for a medicinal chemist (2) to read this without at least a chuckle. The time between HIV was first discovered (1981), and Roche's groundbreaking drug saquinavir was approved was 14 years. Similarly, for hepatitis C (1989), it was 22 years until Merck's boceprevir and Vertex's telaprevir, the first direct-acting hep C drugs were approved.
Zimmer quotes Dr. Anthony Fauci's entirely reasonable description of how such a drug might be used against COVID: "I wake up in the morning, I don’t feel very well, my sense of smell and taste go away, I get a sore throat... I call up my doctor, and I say, ‘I have Covid and I need a prescription." But then this:
Dr. Fauci’s support for research on antiviral pills stems from his own experience fighting AIDS three decades ago. In the 1990s, his institute conducted research that led to some of the first antiviral pills for H.I.V., “protease inhibitors” that block an essential virus protein and can keep the virus at bay for a lifetime.
This is, charitably, misleading and, more accurately, a bunch of nonsense. Saquinavir, the first HIV protease inhibitor, was approved by the FDA in 1995, after which the number of AIDS deaths declined for the first time. Saquinavir (trade name Invirase) was discovered by Roche. The company's first patent for the drug was filed in 1988, meaning that the time invested by the company from discovery to approval was roughly one decade (3).
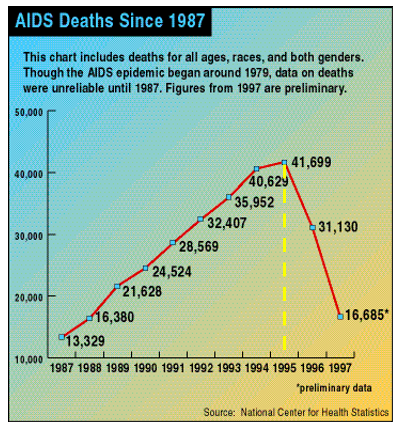
AIDS deaths in the U.S. began to decline for the first time following the approval of Roche's saquinavir (Invirase) in 1995. Source: Wikipedia
Ironically, an article about the approval of saquinavir demonstrated the anti-pharma bias of the Times more than 25 years ago (!):
"The drug, saquinavir, manufactured by Hoffmann-La Roche Inc. of Nutley, N.J., is a member of the new class of drugs called protease inhibitors" (emphasis added)
No, Roche did not simply manufacture the drug. The company discovered and manufactured it: a ridiculous and probably intentional oversimplification.
Neither Did Hepatitis C Drugs
Zimmer's article is similarly biased when it comes to the discovery of drugs that now cure hepatitis C:
"In the early 2000s, researchers found that an antiviral called sofosbuvir could cure hepatitis C close to 100 percent of the time."
Seriously? Researchers found an antiviral drug? Was it hidden away in a closet all this time? This sentence could hardly be more misleading. The discoverers of the hepatitis C virus, who shared the Nobel Prize in 2020, were from both government and academic labs. But this pioneering work, which took place in the late 1980s, would have been for naught had drugs not been developed to treat the infection. And this took a very long time.
The initial discovery was followed by more than two decades of intense research conducted by dozens of drug companies. In total, 77 experimental drugs – an insanely large number – made it into human clinical trials. All of them failed, with the exception of boceprevir and telaprevir in 2011 and then sofosbuvir (brand name Sovaldi) in 2013 (4). One can only imagine how many billions of dollars were incinerated during 20+ years of unsuccessful research.
In the absence of drugs, the overwhelming majority discovered by drug companies, HIV would not be under control, and hepatitis C not easily curable. I'm betting that the 2020 Nobel Prize for Medicine would be going elsewhere.
Will The New York Times ever drop its biases and report the uncomfortable truth about where drugs really come from?
Don't bet on it.
NOTES:
(1) Low molecular weight is another term for small molecules, which can usually be given orally. The upper limit of molecular weight for oral drugs is typically in the 500-1000 range, although there are exceptions.
(2) A medicinal chemist is typically a synthetic organic chemist by training. Then these skills, usually in an industrial setting, are applied to drug discovery research,
(3) This is an educated guess, Patents are usually filed a few years after a discovery (although this can vary widely). I added three years to the seven years between the saquinavir patent filing and its approval.
(4) Despite being first out of the gate, neither boceprevir nor telaprevir were commercial successes. When Sovaldi was approved in 2013, they both became rapidly obsolete. A half-dozen hep C drugs have been approved since Solvaldi.



