One of the most famous passages from the Bible is Ecclesiastes 3:1-8, which inspired the Byrds' song Turn Turn Turn. Here is an excerpt:
There is a time for everything,
and a season for every activity under the heavens:a time to be born and a time to die,
a time to plant and a time to uproot,
a time to kill and a time to heal,
a time to tear down and a time to build...
In his most recent article, my colleague Dr. Chuck Dinerstein could have added his own line:
A time for the kitchen sink, and a time for randomized controlled trials.
It's not nearly as catchy, but it's incredibly important. Dr. Dinerstein was referring to the ongoing COVID-19 pandemic. His thoughtful conclusion was that, at the beginning of the pandemic when we had little idea of what was going on, throwing random drugs and the "kitchen sink" at the coronavirus was a totally legitimate strategy. But now that we have better data, it no longer is. Now, it's a time for randomized controlled trials.
FDA Might Grant Emergency Use for COVID-19 Vaccine Before Clinical Trials Are Finished
That's what makes the FDA Commissioner Stephen Hahn's announcement that his agency may grant emergency use for COVID-19 vaccines before Phase 3 clinical trials are complete so disturbing. To see why, consider how clinical trials typically operate:
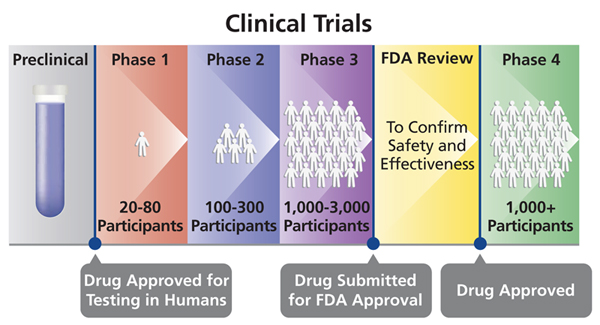
Source: NIH
Once a vaccine passes Phase 1 and Phase 2, scientists have a pretty good idea of how safe and effective it is. But Phase 3 is the real test. The reason is because usually tens of thousands of volunteers are tested in a randomized controlled trial (i.e., some get the vaccine, some do not). Not only does this assess efficacy, it also roots out rare side effects that probably were not detected in the earlier phases.
Let's say a vaccine has a dangerous but rare side effect in 1 out of every 10,000 people. Phase 1 and 2 trials almost certainly won't find it. There aren't enough volunteers. But it likely would be found in a Phase 3 trial. If 30,000 people enroll, we would expect three of them to exhibit the rare side effect. (FYI: Phase 4 "trials" constitute data collection after the product has gone to market.)
This is why it is profoundly important to finish Phase 3 trials prior to approval. We give vaccines to millions or even billions of healthy people. In this hypothetical example, if all 330 million Americans received the vaccine, we would expect 33,000 people to experience the dangerous side effect. Anti-vaxxers, of course, would capitalize on it, further eroding the public's faith in biomedical science. It would be an unmitigated public health disaster.
We have reason to believe that the COVID-19 vaccine could be dangerous. A vaccine developed against the original SARS virus (a cousin of SARS-CoV-2, which causes COVID-19) actually made the disease worse in animal models. We want to make sure something like that doesn't happen. (Other problematic vaccines from the past include one against RSV, which killed two kids, and a swine flu vaccine that triggered paralysis in 450 people.)
Vaccine Success Rates
Once a vaccine enters phase 3 clinical trials, it has an 85% chance of being approved. That also means that, for whatever reason (due to either lack of efficacy or safety or both), 15% are not. We complete the tests for a reason.
Perhaps it shouldn't be surprising that the FDA Commissioner is getting some blowback. Dr. Eric Topol, a physician-scientist and writer, has called for his resignation if he is unable to follow the data and proper scientific protocols.
As for me, I will say this: As much as I love (and proselytize for) vaccines, I will not accept a vaccine that has not completed Phase 3 clinical trials. That's just plain nuts.




