
The chemical reaction that killed the manager of a Buffalo Wings restaurant in Massachusetts last week is arguably the most fundamental of all chemical reactions: Neutralization – the combination of an acid with a base to form a salt. If you've taken chem lab chances are you ran this experiment - the neutralization of hydrochloric acid by sodium hydroxide (or vice versa).
NaOH (base) + HCl (acid) -------> NaCl (salt) + H2O (water)

Reaction 1. A typical neutralization experiment. pH paper (aka litmus paper) measures the acidity or basicity of a solution.
Photo: Fine Art America
Unfortunately, Ryan Baldera, the restaurant manager, tried to clean up the deadly mixture that formed when an employee poured bleach (strongly basic) onto a floor that was covered with an acidic residue that was left by another cleaning product. Although the chemical reaction that formed the chlorine was also an example of neutralization there was one critical difference: when it was exposed to acid the bleach formed salt and water as above, but it also formed chlorine – a deadly green gas, which was used as a chemical weapon in World War I. (1)
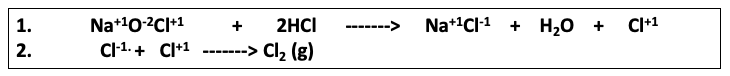
Here's the difference: After the neutralization takes place another reaction – the recombination of Cl+1 and Cl-1 to form Cl2 (chlorine gas) takes place. (2)

Reaction 2. Bleach (sodium hypochlorite) reacts with hydrochloric acid to form salt, water, and chlorine gas. The upward-pointing arrow indicates that a gas has been formed.
News reports indicated that another cleaner called Scale Kleen, a mixture of citric acid and aluminum chloride, had been previously used on the floor. These chemicals, both solids, would have left a film on the floor, similar to the white residue you see on streets (road salt) after a snowstorm. (3) When the bleach solution made contact with the acidic film on the floor the two reacted generating chlorine, as shown in Reaction 2.
Presumably, Mr. Baldera either did not know that Scale Kleen had been used or did not understand the consequences of the chemistry. This caused him to make a serious mistake – one that would cost him his life. According to multiple news reports, Baldera tried to push the foaming mixture out of the store with a squeegee, putting his head near the chlorine that formed. Even a single whiff of chlorine (in sufficient quantity) will cause irreversible and fatal lung damage. Had he left the restaurant with the other people who were evacuated and called 911 there is a good chance that this tragedy would have been averted.
Bleach is an excellent chemical for removing stains and killing germs, but it is chemically reactive. And when it does react it will usually produce chlorine or other toxic gases. Here are some other common chemicals found in homes and also react with bleach:
- Ammonia reacts to form another toxic gas called chloramine (NH2Cl).
- Vinegar (dilute acetic acid) reacts with bleach just like hydrochloric acid, liberating chlorine gas.
- Alcohol reacts with bleach to form a mixture of dangerous gases, including chloroform and hydrogen chloride.
The take-home message from this awful accident is that anytime you are using bleach and notice a color change (especially green), gas being formed, or a swimming pool-like smell, leave the area immediately and call 911. Hazmat crews know exactly what to do. So do chemists.
NOTES:
(1) I originally wrote that Mr. Baldera poured the bleach onto the floor. This apparently was incorrect, as subsequent reporting made clear. According to a senior fire official, a different employee poured the bleach and Mr. Baldera was stricken when trying to clean it up. Thanks to Alyssa Vaughn of Boston Magazine for catching the error.
(2) Although it might seem odd to see Cl+1(chlorine in the +1 oxidation state, Cl-1 is far more common and familiar), the chlorine atom in bleach is, in fact, +1. The fatal reaction can be seen as taking place in two discrete steps - neutralization followed by chlorine formation.
(3) It's a bit more complicated. Scale Kleen consists of citric acid, (which itself can react with bleach) and aluminum chloride, which reacts with water to form HCl and aluminum hydroxide. So, the residue on the floor would have been an acidic mixture of citric acid, aluminum hydroxide and whatever HCl might have been trapped in the solid.



