If you say "palladium" to most people they will think music. But a chemist will think "5% palladium on carbon hydrogenation catalyst." As if we need more evidence that chemists are mutants. But this association is not as strange as you might think. Palladium has one special property - the ability to soak up enormous amounts of hydrogen - 900-times its volume (1). This is why it is commonly used as a hydrogenation catalyst (2) - a reaction that virtually all organic chemists have used.
Let's have a palladium party! (Losers only, please)
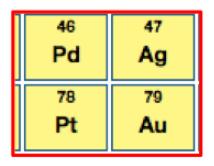
As you can see from its position on the periodic table it has some valuable neighbors, especially platinum and gold.
But you probably wouldn't guess that palladium is the most valuable in the group:
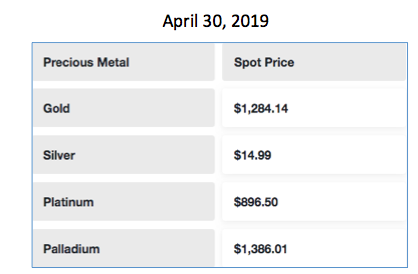
Precious metal prices on April 30th, 2019. Source: GoldLine
Your unwanted palladium lesson continues...
- Palladium was named after Pallas Athena, the Greek goddess of wisdom.
- Most palladium is mined in South Africa; 80% of it is used in catalytic converters. Here's how it works:
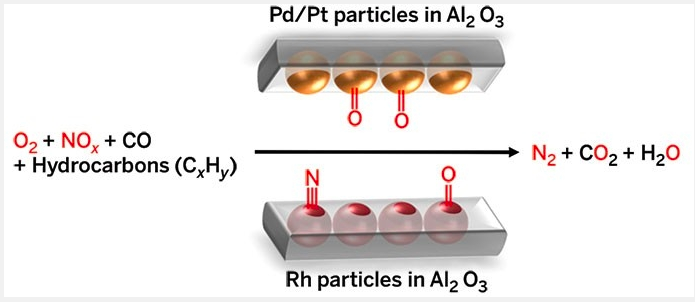
The chemistry of catalytic converters: Three metals (embedded in a matrix of aluminum oxide) make up the typical catalytic converter, which turns dirty engine emissions into clean ones. Each metal has a purpose.
Step 1. Nitric and nitrous oxide, both pollutants, are converted into harmless gases.
Rhodium soaks up the nitrogen oxides. Then platinum splits the oxygen-nitrogen bonds (a reduction reaction), forming harmless nitrogen and oxygen.
(nitric oxide) 2NO ---> N2 + O2 (nitrous oxide) 2N2O ---->N2 + 2O2
Step 2. The palladium catalyzes the oxidation of carbon monoxide and hydrocarbons - two primary sources of air pollution.
2CO + O2 ---> 2CO2
CxHy + O2 ----> CO2 + H2O
One way how chemistry has changed the world:

Downtown Los Angeles 1980 (Left) 2015 (Right). Photo Credits: Philip James Corwin, Corbis (left) Ringo Chiu, Zuma Wire/Corbis (right)
That's plenty of chemistry for one sitting, no? But wait, there's more!
- Like platinum and gold, palladium is called a "noble metal" because it is unreactive in both the environment and (with a few exceptions) in a chemistry lab.
- Unlike some of the elements I've previously described (3), palladium-containing minerals are not gorgeous. They are rather ordinary. This is because the metal doesn't react with environmental sulfur, oxygen, or the other elements that give minerals their colors, such as those we see with other metals, such as copper, iron, and vanadium.

Palladium minerals. Zzz. Photos: e-Rocks.com
A few more...
- Palladium was once used to treat tuberculosis. I have no idea why. Maybe Joe Mercola made that up.
- Palladium is one of the rarest elements in the earth's crust. There is 68 million times more iron, 460 million times more silicon, and 788 million times more than oxygen.
And a reward for any of you who haven't died from boredom. A pretty cool photo...

Jagger and the Stones at the Palladium in New York, 1978. Photo: Sheri Lynn Behr Photography. Hard to miss the attitude.
NOTES:
(1) This property makes the metal useful for metal-hydride batteries and also for capturing hydrogen, deuterium, and tritium generated in nuclear reactors.
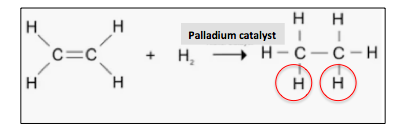
(2) Hydrogenation means adding hydrogen to a molecule. A simple example is the conversion of ethylene to ethane. The added hydrogen atoms are shown in red circles.
(3) See V Is For Vanadium: Versatile, Valuable, And Very Colorful, Copper: A Seriously Cool Element — Especially If You Like Colors And Money



