The expiration dates on drugs can be arbitrary and tell us little about whether the drug in the bottle is still OK to use. Most drugs remain just as potent for years, or even decades, after the expiration date on the bottle. This does not mean that you should disregard the dates; some of them are there for a valid reason, especially antibiotics (1) or drugs in liquid form (2). But an organic chemist can take a look at the chemical structure of a drug and make a pretty good guess about whether the drug in a dry tablet or capsule will decompose or remain intact.
But there are other exceptions. Aspirin (acetylsalicylic acid) is one drug that any chemist would predict, based on the chemical structure alone, to be relatively unstable. This is because aspirin belongs to a class of organic molecules called esters. Esters are formed when alcohols react with carboxylic acids; a molecule of water is lost in the process. Here's a simple example (Figure 1).
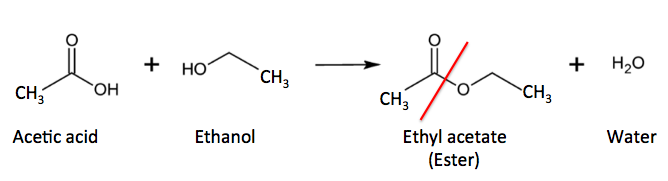
Figure 1. Acetic acid (a carboxylic acid) reacts with ethanol (an alcohol) to form ethyl acetate (an ester) and water The red line shows the new ester bond.
But ester formation is reversible; esters react slowly with water to give back the same alcohol and carboxylic that they were formed from. This is an example of a hydrolysis reaction - water reacting with a substrate (usually an ester or amide) to break it into two fragments. (Figure 2).
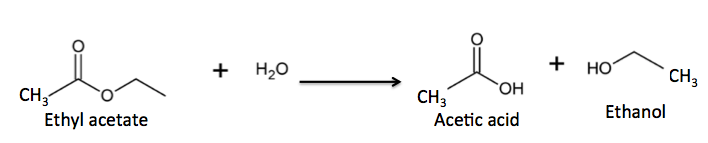
Figure 2. The hydrolysis (reaction with water) of an ester to give a carboxylic acid and alcohol.
It is this second reaction that makes aspirin go "bad" (Figure 3). If moisture has gotten into the bottle some of the aspirin will react with water to give back salicylic acid and acetic acid (vinegar).
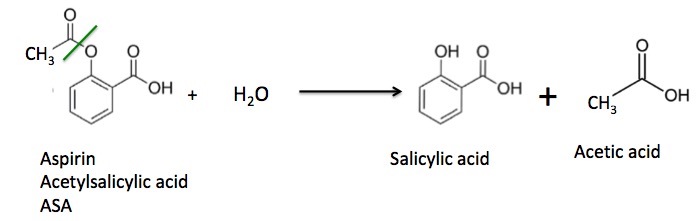
Figure 3. Aspirin reacts slowly with water to give salicylic acid and acetic acid. The green line shows the ester bond that is broken during the hydrolysis reaction.
So, when you open an old bottle of aspirin it is common to smell vinegar. This means that at least some of the aspirin has degraded. But does it matter? Maybe not.
Humans can smell vinegar at a concentration of 0.03 ppm in air (0.000003%). It would take only a tiny fraction of a pill to go bad to generate enough vinegar that would be detectable by the human nose. So, even if more than 99.99% of the aspirin remained intact you would still smell vinegar. But, suppose the bottle was really old? Would it matter? Not that much.
Vinegar is not only harmless but red wine vinegar is now advertised as a cure-all for all human ailments. So that isn't going to hurt you. What about the other part of the aspirin molecule - salicylic acid. Will this hurt you? Not really, especially since it is sold right next to aspirin on pharmacy shelves (3).
Doan's Pills contain salicylic acid, magnesium salt.
Salicylic is chemically similar to aspirin but not nearly as effective. For some reason, it is sold as an analgesic remedy for back pain. Talk about ridiculous. A very weak version of aspirin is sold specifically for lower back pain as if your back knows that it's supposed to work. The only reason I can imagine why it is still sold is that it has been used forever.
 \
\
Doan's Pills ca. 1950. Photo: The National Museum of American History.
If you want to throw out old aspirin, fine. But its degradation products are both touted as health remedies.
NOTES:
(1) Most antibiotics lose potency with time because of degradation. The tetracycline class is notorious for this.
(2) Solids don't normally react unless they are in solution. This is why dry capsules and tablets are more stable than liquid solutions and syrups.
(3) Salicylic acid is more irritating to the stomach and is not well absorbed. This is why it is not a very good drug.




