“The dance of accommodation” – the back and forth between diagnosis and therapy, one building on the other, intwined, Ginger and Fred. Are Small Breast Cancers Good because They Are Small or Small because They Are Good?, [1] by Lannin and Wang, has a lot to teach us about that dance.
“But let us not be charmed by the imagery of a perfect dance in which both partners execute their steps flawlessly, where each communicates to the other just what nuances and modifications are about to take place, and the other comprehends elegantly, making the transitions creatively and without missing a beat. Such dancing many occur in the movies, but seldom in real life.” [2]
Caring for disease begins with diagnosis, you cannot treat a problem that is unknown to you. The diagnosis of breast cancer begins with identifying “a lump” whether detected by the patient or by screening mammography which detects more smaller lumps because it is more sensitive in finding the proverbial ‘needle in the haystack.’ Large lumps are easy to identify, no improvement is necessary. Because our science showed that as cancer got larger, it became ‘more aggressive,' we sought to find lumps earlier before the situation becomes more difficult or hopeless. Our ability to detect these smaller cancers increased over about eight years from 25.3 per thousand to 34.7 per thousand – about a 37% improvement [3]. In the words of the study’s author, “The change likely reflects improvements in mammography imaging technology, which permit the visualization of smaller lesions and greater detection of calcifications that result in increased cancer detection.” The underlying assumption was that earlier detection results in a better outcome and the measure of early was size. Therapy informed diagnosis and diagnosis responded. It wasn’t simply that size mattered, but for mammography, it was all that could be measured. Once identified, the lump biopsied, cancer confirmed and treatment initiated.
The treatment options were almost entirely surgical, with chemotherapy and radiation playing a supporting, adjunctive role. While ‘cure’ might be allusive, we found that early intervention resulted in improved survival, five and then ten-year cures. But for newer treatments, where chemotherapy or radiation was primary or sole treatment, our prior success necessitated a longer time-period to see better outcomes. Treatment changed at one rate, diagnosis at another, the dancers were out of sync. Another important treatment discovery was our failure to treat all small lumps successfully. Categorizing lumps by size was too heterogeneous for treatment. Size does not precisely identify biologic behavior. As best we can tell, that behavior is more closely associated with cancer's response to hormones. From treatment’s viewpoint, we now want to characterize cancers by their behavior; size is no longer the best measure. Those new criteria, because of the lag time brought by extended survival, was not established and shared for some time.
And that brings us to this current study. With the advantage of hindsight, the authors correlated the size of breast cancer at detection with the three markers we use to measure biological behavior, the grade (increasing abnormal appearance of the cells), and the response to estrogen and progesterone. This graph, from the study, captures the essence of their findings.
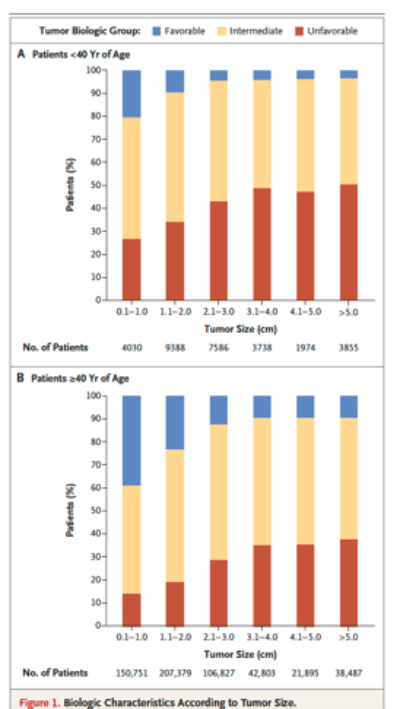
If you look across the categories of tumor size, the percentage of cancers with favorable biologic behavior decrease. Proof that size does matter. But when you look at the individual columns you see that size is heterogeneous regarding behavior. Some small tumors are biologically very aggressive (unfavorable in the chart) and others not so much. And the proportion of favorable tumors increases for patients over 40, the group of patients most likely to be diagnosed with cancer. The authors conclude, “Both tumor size and biologic features influence prognosis, but frequently a large favorable tumor can have a better prognosis than a small unfavorable tumor.” And nod to the future of diagnostic/prognostic factors, recognizing that “grade and receptor status are rather crude predictors of biologic features as compared with molecular assays.” Bottom line, size has lost its primary prognostic role, challenged by grade and hormone receptor status, measures that mammography cannot identify. All our effort in improving mammography to detect small has increased our difficulties [4] because our diagnostic tool is no longer dancing with our therapeutic considerations.
“Moments can come when our interactions can take on the qualities suggested – when the partners are no longer simply dancing, but also communicating about changing the dance itself to make it more satisfying for both.” [2]
For Lannin and Wang, the changing dance lies in the term overdiagnosis. Defined as “An over- diagnosed cancer is one detected by screening that would not have presented clinically during the patient’s lifetime in the absence of screening, i.e., the patient would have died from other causes with preclinical disease. …it does not matter if the tumor would eventually progress, but whether it would progress within the lifetime of the patient.” [5] Treatment should be tailored to the patient - there is no sense in treating a five-year problem in a patient who will die of other causes in a year. But I had always thought of that as judgment, informed by evidence. Overdiagnosis, as a term, raises concerns for me. First, it is presented as a quantitative measure based on how early the diagnosis is made (lead time) in relation to your life expectancy. Unfortunately, neither of these two numbers is known with any precision, so how useful is this pseudo-quantitative measure over judgment? The second difficulty is the term itself; we are not overdiagnosing anything, a breast cancer is present. We are over treating because when stratified by biologic behavior and not size some of these tumors will take 15 years or more to become problematic. And this begins to put breast cancer treatment into the perspective where prostate cancer has lingered for many years. Who do you treat when the tumor is slow-growing, and the life expectancy is shorter than the tumor’s growth? Not a diagnostic issue, a therapeutic one. Lannin and Wang capture the idea here, “Educating physicians, patients, and the public that some cancers are indolent and individualizing treatment algorithms to provide ‘personalized medicine’ will aid in addressing this problem.” Individualizing treatment algorithms is quantitative speak for clinical judgment. I would not be in a rush to believe that people, when told they have cancer that will not kill them, will be content to wait and watch. That hasn’t been the case for prostate cancer.
[1] A recent article in the New England Journal of Medicine. I am sorry that the link only takes you to the exceedingly short abstract, to see the entire article perhaps it is worth using Sci-hub, the DOI is 10.1056/NEJMsr1613680
[2] The Systems Bible John Gall
[3] Taken from a study of mammography’s improvement from the National Cancer Institute’s Breast Cancer Surveillance consortium. Without knowing the underlying cancer rate we do not know how close we are to finding all those cancers (the true sensitivity of mammography scientifically speaking).
[4] The positive predictive value (PPV), “the probability that patients with a positive diagnostic mammogram truly have a malignancy,” the test’s specificity has fallen as more small lumps were identified, increasing the background diagnostic noise. In the same period as mammography’s improved detection of small, the PPV fell by about 12%.
[5] Etzioni R, Xia J, Hubbard R, Weiss NS, Gulati R. A reality check for overdiagnosis estimates associated with breast cancer screening. J Natl Cancer Inst 2014; 106: 106