
Useless trivia item for a Wednesday:
Whether you are choosing 87, 89, or 93-octane rated gasoline, you're not buying octane. Why? Because if you were actually putting octane into your car, it would screw it up big time.
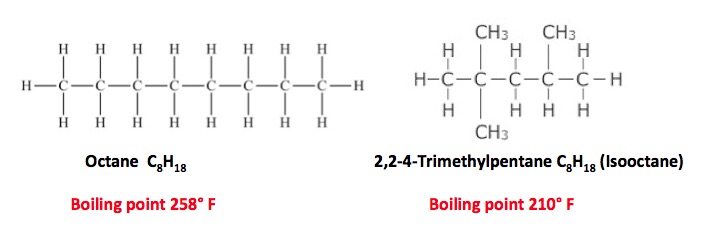
The "octane" rating of gasoline actually measures the amount of an additive called isooctane. The two are isomers- they have the same chemical formula but different structures and properties. Octane is a chain of eight contiguous carbon atoms. Isooctane has chain of five contiguous and three "branched" carbon atoms. Both are hydrocarbons. The branching is why cars need it. To burn fuel properly.
In an engine cylinder, fuel are air are compressed, and then the mixture is ignited by a spark plug. But, straight chain hydrocarbons have an annoying tendency to ignite too soon (before they reach the spark). This causes a pinging sound - engine knock. Engine knock causes very high pressure inside the cylinders, which can damage the engine. Branched chain hydrocarbons are "better behaved." They hang around long enough to get the timing right. So, the higher the percentage of isooctane, the less the engine will knock.
There are other examples of branched fuel additives, the most notorious being tetraethyllead. Note that the entire molecule is one big branch.
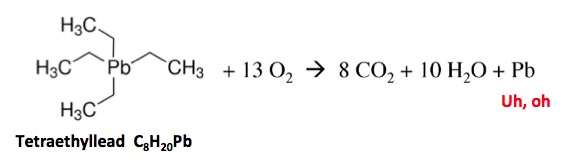
But tetraethyllead has a disturbing tendency to give off tiny particles of lead metal upon combustion, and plenty of them. In 1965, a total of 250 metric tons of tetraethyllead was used in gasoline, which means that burning it released 78 tons of lead into the atmosphere. Not good. Lead-based fuels were banned in the US in 1996.
So, without icky lead, how do you stop engine knock? The next idea wasn't all that bad—getting rid of the lead by substituting methyl t-butyl ether (1,2) (MTBE), but it didn't work out as expected. There were two problems with this substitute, which began to replace tetraethyllead in 1979, peaked in the 1990s and was gone by 2006.
.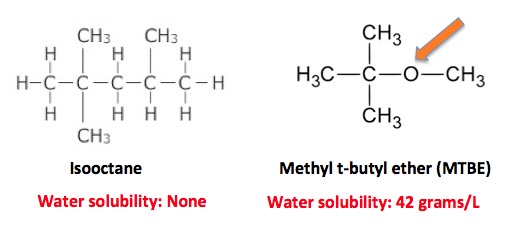
First, the oxygen in the molecule enables MTBE to dissolve in water. Hydrocarbons, which don't contain oxygen (orange arrow), are insoluble. So, when MTBE was spilt, some of it would make its way down to the water table and stay there as a water solution. (Hydrocarbons will just evaporate.) MTBE does not readily biodegrade, so once it's in a well or public water supply, it stays around. Until it is consumed. Then your liver breaks it down:

Breakdown of MTBE by liver enzymes
There is an interesting lesson in toxicology here. MTBE is not genotoxic (does not damage DNA) in the Ames test, so there is no reason to expect that it would be carcinogenic. But when the test was run in the presence of liver cells, the Ames test turned positive. Formaldehyde was shown to be the metabolite that was responsible for mutagenicity of MTBE.
As I have written (here and here), not only are trace amounts of formaldehyde not harmful to people, but it is also a required building block for the biosynthesis of several amino acids. The body is well equipped to handle small amounts of formaldehyde. It lasts about a minute in blood, after which it is converted to formic acid and carbon dioxide.
MTBE quickly fell out of favor because of storage tank leakage. In areas near leaky tanks, ground water contained measurable amounts of the chemical, and reports of water with an unpleasant smell or taste were not uncommon (3). MTBE has been shown to cause cancer when given in high doses to rats, but there are no epidemiological data regarding human health and the additive.
MTBE was banned in 19 states between 2000 and 2006, and nationwide by 2006. The real reason for the ban isn't entirely clear. Whether it was due to health fears, water taste and smell, the ethanol/corn lobby, or some combination of these, its presence on the scene was short-lived.
Ethanol is a pretty lousy additive too. It is expensive (comes from fermenting corn, so you are burning food), corrosive to certain metal components, is only partially miscible with gasoline (the two components can't be shipped together or the ethanol will come out of solution and sink to the bottom of the tank), and it has a particular affinity for water, so that (especially in cold weather) more water will find its way into your car's gas tank.
So, what's next? Hard to say. All additives have their own baggage (4). Except maybe....
Notes:
(1) You will see the name methyl tertiary-butyl ether used as well. The shorthand— methyl t-butyl ether is perfectly acceptable.
(2) In addition to branching, MTBE also provided oxygen, which helps fuel burn more efficiently, and cuts down on carbon monoxide formation.
(3) We sometimes used it in lab. Pretty stinky.
(4) Other additives called aromatic hydrocarbons (toluene, xylene) can also increase the performance of gasoline, but they have their own toxicity and combustion issues.



