How would you like to spend the day taking water samples from a sewer pipe off the coast of Sardinia? It’s probably not as much fun as it sounds, but it’s a good thing that Giuseppe Brotzu found himself doing just that in 1945.
Brotzu made what turned out to be a very important discovery—a new class of antibiotics called cephalosporins, which includes drugs such as Keflex and Suprax. About 80 different cephalosporins have approved in the US.
Since we are in a (losing) race against bacterial resistance, a new class of antibiotics is just what the doctor ordered. Thanks to a group from Quebec, we may have another avenue to explore.
Finding new classes of antibiotics—something that is sorely needed—is very difficult. When American Council advisor Dr. David Shlaes and I write about the risks associated with improper use of antibiotics, such as use in growth promoters (here and here), we often get the same simplistic answer: "We need more antibiotics," as if this is just a matter of dropping coins in a vending machine. Well, it's not. It's anything but, which can be illustrated by the efforts of a Canadian team of natural product scientists (1).
Claudia Carpentier of Université Laval in Quebec and colleagues did not need to sit around a sewer pipe, but they probably had to bundle up. The scientists were exploring northern region of Quebec to collect samples of the lichen Stereocaulon paschale. This species of lichen is endangered because shrubs were overrunning over the area, and changing the ecosystem where the lichens grow. The group wanted to study the phytochemicals contained in the lichen while it still existed.
The Stereocaulon was ground up to give a crude extract, which was found to contain 13 chemical compounds (2). Of the 13, 11 had been previously isolated from other lichens, but two of were novel (never before isolated and characterized).
All 13 components were tested against two species of bacteria: Porphyromonas gingivalis, which causes periodontal disease, and Streptococcus mutans—the primary bacteria in the mouth, which causes tooth cavities, as well as bacterial endocarditis—an infection of the heart, which can be deadly (3).
Three of the 13 showed modest antibacterial activity. It should come of no surprise that antimicrobial compounds were found in nature. Most classes of antibiotics, such as penicillins, macrolides, and tetracyclines, have been derived from living organisms (4).
Stereocaulon paschale. Source: Journal of Natural Products
How well did the lichen chemicals do? Not so great. The table below shows the antibacterial activity (or lack thereof) of all 13 against Porphyromonas gingivalis and Streptococcus mutans. The term MIC (minimum inhibitory concentration) is the lowest concentration at which the bacteria stop growing. The term MIB (minimal bactericidal concentration) is the lowest concentration at which the bacteria are killed. The lower the number, the more potent the antibiotic. This is where you might not be likin' lichens. Although compounds 11 and 13 (orange circles) do possess some antibacterial properties, it is modest at best. The most compound is still five-to-ten-times less so than the positive control, Penicillin G (5).
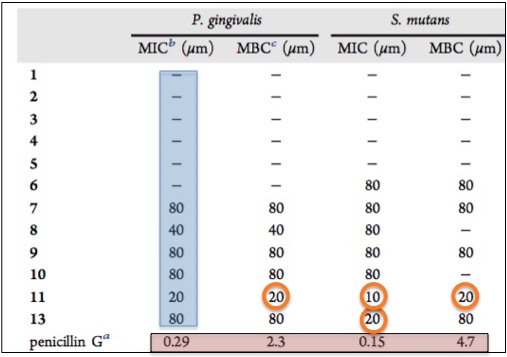
Antibacterial activity of Dibenzofurans and Pseudodepsidones isolated from Stereocaulon paschale Penicillin G is used as a positive control.
Concentrations are expressed in micromolar.Source: Journal of Natural Products, 2017; DOI: 10.1021/acs.jnatprod.6b00831.
It is very unlikely that compounds with such high MICs would ever become drugs. The dose needed to achieve adequate blood levels would be enormous. Two of them look like they could be a starting point, because when low potency compounds are put into the hands of medicinal chemists, it is almost a certainty that they will be able to improve the potency. Depending on the original compound and the disease or infection, making an improvement of 10-times is usually trivial. Increases of hundred- or even thousand-fold are not uncommon. Even the smallest of changes in molecular structure can make a huge difference in potency:

Impact of the addition of a methyl group on potency against two cancer targets
Whether the discovery of three natural products having low-level antibacterial activity will ever yield a drug remains to be seen. The three are not potent enough, but this could change entirely once chemists get their hand on them. This is just one more example of what can be accomplished with nature and science work together (See: Semisynthetic: A *Real* Word That Saves Lives").
Notes:
(1) Natural products chemistry is one of several methods used to discover new drugs. It involves testing crude extracts from an enormous number of living organisms (mostly plants) and testing these extracts to see if there is any of activity against molecular targets of different diseases or infections. Although many drugs have been discovered this way, it is very difficult.
(2) This is both challenging and time consuming. The crude extract had to be separated to isolate each the resulting 13 compounds, which had to be purified and their chemical structures determined.
(3) Dentists and endodontists are now paying more attention to tooth and gum health than in the past because of the risk of heart infection arising from oral infections of Streptococcus mutans.
(4) Although antibiotics usually come from living organisms, the original discovery may or may not end up in the pharmacy. Instead, synthetically modified versions that are more potent, or have improved properties will often become the drug(s) in a given series.
(5) Early Penicillins were distinguished from each other by using letters. In this case G is shorthand for benzylpenicillin. G means "gold standard"
Original Source: Claudia Carpentier, et. al. "Dibenzofurans and Pseudodepsidones from the Lichen Stereocaulon paschale Collected in Northern Quebec" Journal of Natural Products, Publication Date (On line): January 12, 2017.



