Besides making wigs, or perhaps some rather bizarre clothing and artwork, there aren't a lot of practical uses for discarded human hair. But that could change thanks to a team of Japanese and South Korean chemists.
Published in the journal ACS Sustainable Chemistry & Engineering, the team has demonstrated the use of human hair to grow catalytic nanoparticles (i.e., nanoparticles that are capable of carrying out chemical reactions).
Why human hair? Nanoparticles, like crystals, often need to be grown on something, particularly if they are going to be immobilized for the purpose of carrying out chemical reactions. Various support structures have been used, from wood to bacterial cell walls, but many are not ideal. Some are too expensive, and others require modification with toxic chemicals. On the other hand, human hair is abundant, cheap, and biodegradable. This combination makes it suitable for so-called "green chemistry," which places an emphasis on sustainability and the use of environmentally friendly compounds.
The researchers collected virgin human hair (no, not hair from a virgin, but virgin as in "chemically untreated") and ground it into a fine powder. The hair's proteins were extracted, and solutions containing either silver or gold ions were added. Mixing in sodium borohydride, a reducing agent, triggered the formation of silver or gold nanoparticles on the hair proteins. (See figure.)
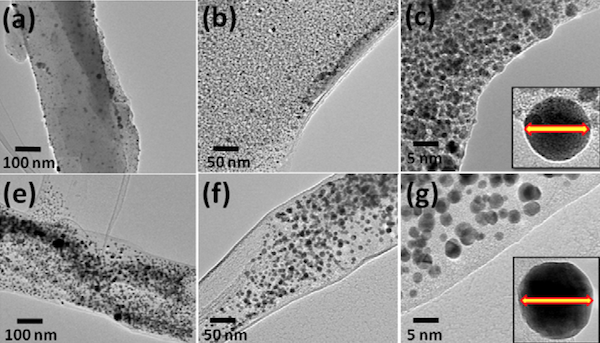
The images above depict silver (a, b, c) and gold (e, f, g) nanoparticles at increasing magnification. The final image in each row depicts that the average nanoparticle had a diameter of about 3 nanometers, or 3 billionths of a meter. (To put that into perspective, silver and gold nanoparticles are often at least 10 times bigger.)
After synthesis, the researchers filtered and dried their protein-nanoparticle catalysts. Then, they showed the utility of their synthetic method by demonstrating the efficiency with which the catalysts performed industrially important chemical reactions. The authors believe that this efficiency was due largely to the tiny size (but large surface area) of their nanoparticles. Moreover, their catalysts were reusable.
Who knew the barber shop floor was covered with such valuable materials?
Source: Dian Deng, Mayakrishnan Gopiraman, Seong Hun Kim, Ill-Min Chung, and Ick Soo Kim. "Human Hair: A Suitable Platform for Catalytic Nanoparticles." ACS Sustainable Chem. Eng. Article ASAP. Published: 31-Aug-2016. DOI: 10.1021/acssuschemeng.6b01689




